Age and Human Regenerative Capacity Impact of Cardiovascular Risk Factors
- PMID: 27436845
- PMCID: PMC5026592
- DOI: 10.1161/CIRCRESAHA.116.308461
Age and Human Regenerative Capacity Impact of Cardiovascular Risk Factors
Abstract
Rationale: We investigated aging of human endogenous reparative capacity and aimed to clarify whether it is affected by presence of cardiovascular disease or its risk factors (RFs).
Objective: Circulating progenitor cell (PC) levels reflect endogenous regenerative potential. The effect on PC of healthy aging compared with aging with RFs or cardiovascular disease (CVD) is unknown. We examined whether exposure to RF and CVD leads to an accelerated decline in circulating PC with increasing age.
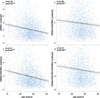
Methods and results: In 2792 adult subjects, 498 were free of RFs (smoking, diabetes mellitus, hypertension, or hyperlipidemia), 1036 subjects had 1 to 2 RF, and 1253 had ≥3 RFs or CVD. PC were enumerated by flow cytometry as CD45(med+) mononuclear cells expressing CD34 and subsets coexpressing CD133, CXCR4, and vascular endothelial growth factor receptor-2 epitopes. Younger age, male sex, and larger body size correlated with higher PC counts (P<0.01). After multivariable adjustment, both age and RF categories were independently associated with PC counts (P<0.05), with lower PC counts in older subjects and those with higher RF burden or CVD. PC counts remained unchanged with increasing age in healthy individuals. There were significant interactions between age and RF categories (P≤0.005), such that for younger subjects (<40 years), RFs were associated with increased PC counts, whereas for older subjects (>60 years), RFs and CVD were associated with lower PC counts.
Conclusions: Circulating PC levels do not decline with healthy aging; RF exposure at a younger age stimulates PC mobilization, whereas continued exposure is associated with lower PC levels in later life. Over the lifespan, exposure to RFs and CVD is associated with an initial stimulation and subsequent decline in circulating PC levels, which reflect endogenous regenerative capacity.
Keywords: aging; cardiovascular risk factors; progenitor cells.
© 2016 American Heart Association, Inc.
Figures
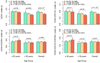

Comment in
-
New Definition of Aging? Measuring Regenerative Capacity in Patients.Circ Res. 2016 Sep 16;119(7):774-5. doi: 10.1161/CIRCRESAHA.116.309622. Circ Res. 2016. PMID: 27635077 No abstract available.
-
Endothelial progenitor cells and cardiovascular risk: does ageing trump all other factors?Ann Transl Med. 2016 Dec;4(24):553. doi: 10.21037/atm.2016.12.34. Ann Transl Med. 2016. PMID: 28149914 Free PMC article. No abstract available.
Similar articles
-
Sex Differences in Circulating Progenitor Cells.J Am Heart Assoc. 2017 Oct 3;6(10):e006245. doi: 10.1161/JAHA.117.006245. J Am Heart Assoc. 2017. PMID: 28974500 Free PMC article.
-
Progenitor Cells and Clinical Outcomes in Patients With Heart Failure.Circ Heart Fail. 2017 Aug;10(8):e004106. doi: 10.1161/CIRCHEARTFAILURE.117.004106. Epub 2017 Aug 8. Circ Heart Fail. 2017. PMID: 28790053 Free PMC article.
-
Circulating Progenitor Cells and Racial Differences.Circ Res. 2018 Aug 3;123(4):467-476. doi: 10.1161/CIRCRESAHA.118.313282. Circ Res. 2018. PMID: 29930146 Free PMC article.
-
Sex differences in cardiovascular risk factors and disease prevention.Atherosclerosis. 2015 Jul;241(1):211-8. doi: 10.1016/j.atherosclerosis.2015.01.027. Epub 2015 Jan 28. Atherosclerosis. 2015. PMID: 25670232 Review.
-
Cellular senescence in cardiovascular diseases: potential age-related mechanisms and implications for treatment.Curr Pharm Des. 2013;19(9):1710-9. Curr Pharm Des. 2013. PMID: 23061728 Review.
Cited by
-
Psychosocial stress and cardiovascular disease.Am J Prev Cardiol. 2025 Mar 19;22:100968. doi: 10.1016/j.ajpc.2025.100968. eCollection 2025 Jun. Am J Prev Cardiol. 2025. PMID: 40225054 Free PMC article. Review.
-
Stress and cardiovascular disease: an update.Nat Rev Cardiol. 2024 Sep;21(9):603-616. doi: 10.1038/s41569-024-01024-y. Epub 2024 May 2. Nat Rev Cardiol. 2024. PMID: 38698183 Review.
-
Transplantation of CD34+ cells for myocardial ischemia.World J Transplant. 2021 May 18;11(5):138-146. doi: 10.5500/wjt.v11.i5.138. World J Transplant. 2021. PMID: 34046316 Free PMC article. Review.
-
Endothelial progenitor cells and cardiovascular risk: does ageing trump all other factors?Ann Transl Med. 2016 Dec;4(24):553. doi: 10.21037/atm.2016.12.34. Ann Transl Med. 2016. PMID: 28149914 Free PMC article. No abstract available.
-
The association between baseline circulating progenitor cells and vascular function: The role of aging and risk factors.Vasc Med. 2022 Dec;27(6):532-541. doi: 10.1177/1358863X221116411. Epub 2022 Sep 5. Vasc Med. 2022. PMID: 36062298 Free PMC article.
References
-
- Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–228. - PubMed
-
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. - PubMed
-
- Gehling UM, Ergun S, Schumacher U, Wagener C, Pantel K, Otte M, Schuch G, Schafhausen P, Mende T, Kilic N, Kluge K, Schafer B, Hossfeld DK, Fiedler W. In vitro differentiation of endothelial cells from ac133-positive progenitor cells. Blood. 2000;95:3106–3112. - PubMed
-
- Urbich C, Dimmeler S. Endothelial progenitor cells: Characterization and role in vascular biology. Circulation research. 2004;95:343–353. - PubMed
-
- Kawamoto A, Iwasaki H, Kusano K, Murayama T, Oyamada A, Silver M, Hulbert C, Gavin M, Hanley A, Ma H, Kearney M, Zak V, Asahara T, Losordo DW. Cd34-positive cells exhibit increased potency and safety for therapeutic neovascularization after myocardial infarction compared with total mononuclear cells. Circulation. 2006;114:2163–2169. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

