Cell division plane orientation based on tensile stress in Arabidopsis thaliana
- PMID: 27436908
- PMCID: PMC4968720
- DOI: 10.1073/pnas.1600677113
Cell division plane orientation based on tensile stress in Arabidopsis thaliana
Abstract
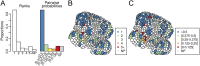
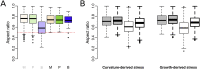
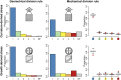
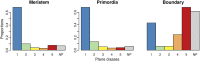
Cell geometry has long been proposed to play a key role in the orientation of symmetric cell division planes. In particular, the recently proposed Besson-Dumais rule generalizes Errera's rule and predicts that cells divide along one of the local minima of plane area. However, this rule has been tested only on tissues with rather local spherical shape and homogeneous growth. Here, we tested the application of the Besson-Dumais rule to the divisions occurring in the Arabidopsis shoot apex, which contains domains with anisotropic curvature and differential growth. We found that the Besson-Dumais rule works well in the central part of the apex, but fails to account for cell division planes in the saddle-shaped boundary region. Because curvature anisotropy and differential growth prescribe directional tensile stress in that region, we tested the putative contribution of anisotropic stress fields to cell division plane orientation at the shoot apex. To do so, we compared two division rules: geometrical (new plane along the shortest path) and mechanical (new plane along maximal tension). The mechanical division rule reproduced the enrichment of long planes observed in the boundary region. Experimental perturbation of mechanical stress pattern further supported a contribution of anisotropic tensile stress in division plane orientation. Importantly, simulations of tissues growing in an isotropic stress field, and dividing along maximal tension, provided division plane distributions comparable to those obtained with the geometrical rule. We thus propose that division plane orientation by tensile stress offers a general rule for symmetric cell division in plants.
Keywords: Arabidopsis; cell division plane; mechanical forces; meristem; vertex model.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








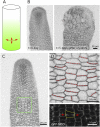


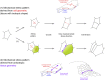
Comment in
- doi: 10.1073/pnas.1609309113
References
-
- Hofmeister W. Zusatze und berichtigungen zu den 1851 veröffentlichen untersuchungen der entwicklung höherer kryptogamen. Jahrbucher fur Wissenschaft und Botanik. 1863;3:259–293. German.
-
- Sachs J. Uber die Anordnung der Zellen in jungsten Pflanzentheilen. Arbeiten des Botanisches Institut Wurzburg. 1878;2:46–104. German.
-
- Errera L. Über Zellformen und Seifenblasen. Botanisches Centralblatt. 1888;34:395–398. German.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

