RNAseq reveals hydrophobins that are involved in the adaptation of Aspergillus nidulans to lignocellulose
- PMID: 27437031
- PMCID: PMC4950808
- DOI: 10.1186/s13068-016-0558-2
RNAseq reveals hydrophobins that are involved in the adaptation of Aspergillus nidulans to lignocellulose
Abstract
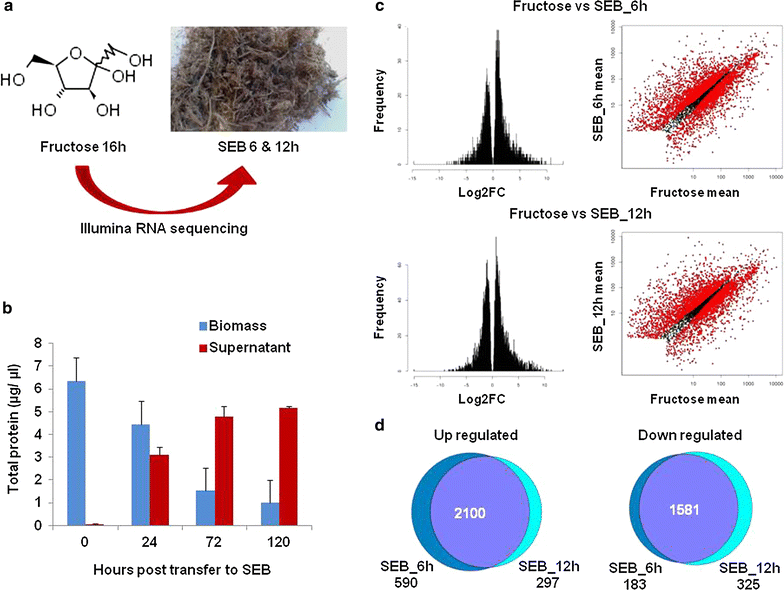
Background: Sugarcane is one of the world's most profitable crops. Waste steam-exploded sugarcane bagasse (SEB) is a cheap, abundant, and renewable lignocellulosic feedstock for the next-generation biofuels. In nature, fungi seldom exist as planktonic cells, similar to those found in the nutrient-rich environment created within an industrial fermenter. Instead, fungi predominantly form biofilms that allow them to thrive in hostile environments.
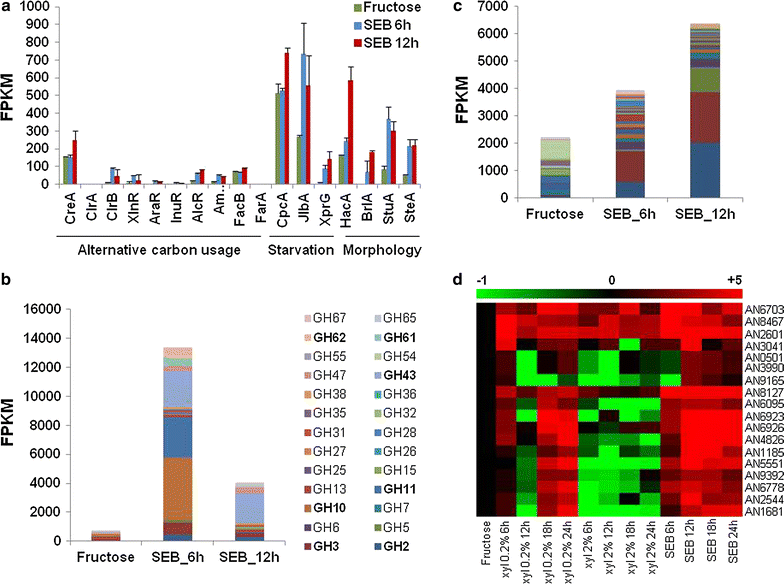
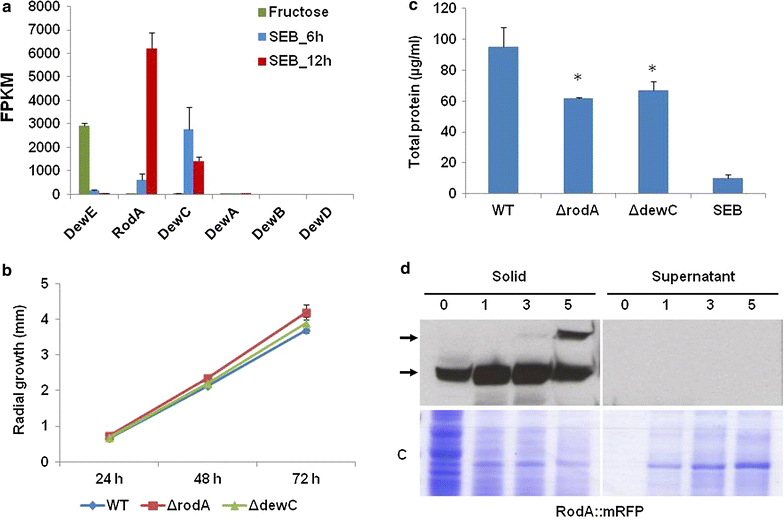
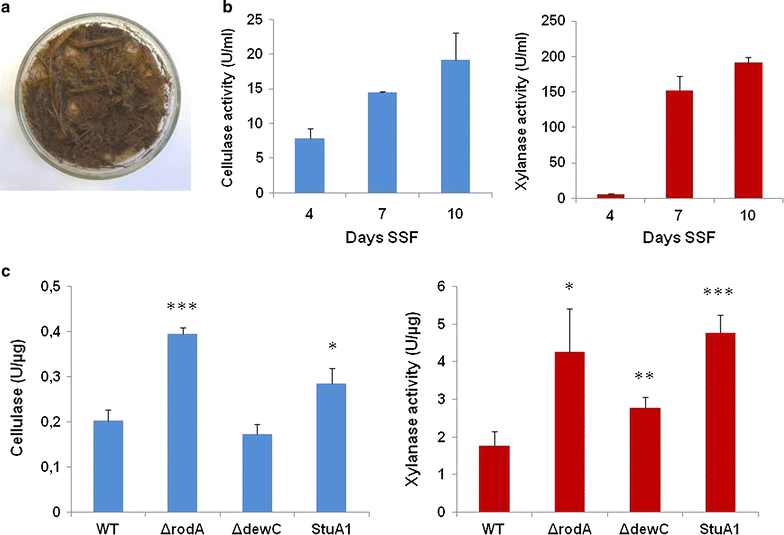
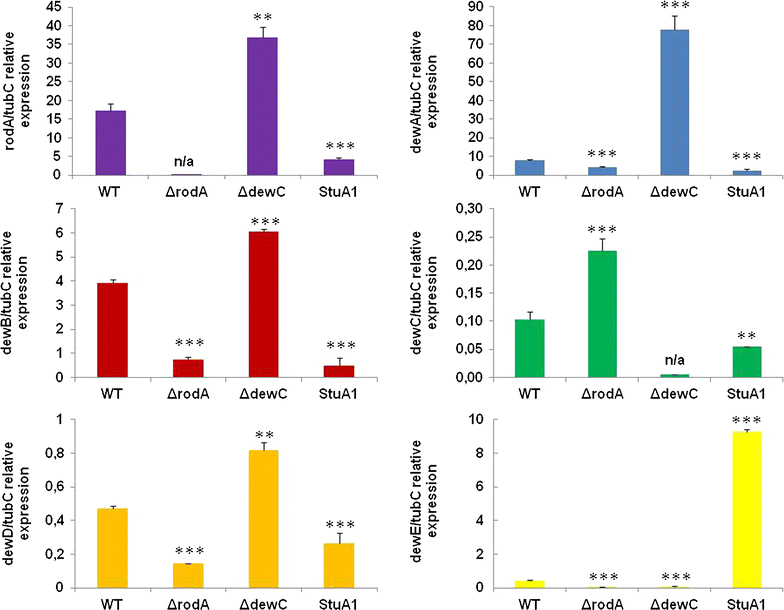
Results: In turn, we adopted an RNA-sequencing approach to interrogate how the model fungus, Aspergillus nidulans, adapts to SEB, revealing the induction of carbon starvation responses and the lignocellulolytic machinery, in addition to morphological adaptations. Genetic analyses showed the importance of hydrophobins for growth on SEB. The major hydrophobin, RodA, was retained within the fungal biofilm on SEB fibres. The StuA transcription factor that regulates fungal morphology was up-regulated during growth on SEB and controlled hydrophobin gene induction. The absence of the RodA or DewC hydrophobins reduced biofilm formation. The loss of a RodA or a functional StuA reduced the retention of the hydrolytic enzymes within the vicinity of the fungus. Hence, hydrophobins promote biofilm formation on SEB, and may enhance lignocellulose utilisation via promoting a compact substrate-enzyme-fungus structure.
Conclusion: This novel study highlights the importance of hydrophobins to the formation of biofilms and the efficient deconstruction of lignocellulose.
Keywords: Biofilm; Fungi; Hydrolytic enzymes; Hydrophobin; Sugarcane bagasse.
Figures

Similar articles
-
Aspergillus Hydrophobins: Physicochemical Properties, Biochemical Properties, and Functions in Solid Polymer Degradation.Microorganisms. 2022 Jul 25;10(8):1498. doi: 10.3390/microorganisms10081498. Microorganisms. 2022. PMID: 35893556 Free PMC article. Review.
-
Comparative transcriptome analysis reveals different strategies for degradation of steam-exploded sugarcane bagasse by Aspergillus niger and Trichoderma reesei.BMC Genomics. 2017 Jun 30;18(1):501. doi: 10.1186/s12864-017-3857-5. BMC Genomics. 2017. PMID: 28666414 Free PMC article.
-
Six hydrophobins are involved in hydrophobin rodlet formation in Aspergillus nidulans and contribute to hydrophobicity of the spore surface.PLoS One. 2014 Apr 10;9(4):e94546. doi: 10.1371/journal.pone.0094546. eCollection 2014. PLoS One. 2014. PMID: 24722460 Free PMC article.
-
Analysis of the ionic interaction between the hydrophobin RodA and two cutinases of Aspergillus nidulans obtained via an Aspergillus oryzae expression system.Appl Microbiol Biotechnol. 2017 Mar;101(6):2343-2356. doi: 10.1007/s00253-016-7979-5. Epub 2016 Dec 5. Appl Microbiol Biotechnol. 2017. PMID: 27917435
-
How nutritional status signalling coordinates metabolism and lignocellulolytic enzyme secretion.Fungal Genet Biol. 2014 Nov;72:48-63. doi: 10.1016/j.fgb.2014.06.012. Epub 2014 Jul 8. Fungal Genet Biol. 2014. PMID: 25011009 Review.
Cited by
-
Aspergillus Hydrophobins: Physicochemical Properties, Biochemical Properties, and Functions in Solid Polymer Degradation.Microorganisms. 2022 Jul 25;10(8):1498. doi: 10.3390/microorganisms10081498. Microorganisms. 2022. PMID: 35893556 Free PMC article. Review.
-
StuA-Regulated Processes in the Dermatophyte Trichophyton rubrum: Transcription Profile, Cell-Cell Adhesion, and Immunomodulation.Front Cell Infect Microbiol. 2021 Jun 8;11:643659. doi: 10.3389/fcimb.2021.643659. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34169004 Free PMC article.
-
Transcriptome Profiling-Based Analysis of Carbohydrate-Active Enzymes in Aspergillus terreus Involved in Plant Biomass Degradation.Front Bioeng Biotechnol. 2020 Oct 6;8:564527. doi: 10.3389/fbioe.2020.564527. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 33123513 Free PMC article.
-
Comparative analysis of surface coating properties of five hydrophobins from Aspergillus nidulans and Trichoderma reseei.Sci Rep. 2018 Aug 13;8(1):12033. doi: 10.1038/s41598-018-29749-0. Sci Rep. 2018. PMID: 30104653 Free PMC article.
-
Analysis of the Transcriptome in Aspergillus tamarii During Enzymatic Degradation of Sugarcane Bagasse.Front Bioeng Biotechnol. 2018 Sep 18;6:123. doi: 10.3389/fbioe.2018.00123. eCollection 2018. Front Bioeng Biotechnol. 2018. PMID: 30280097 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

