Clinical Decision Support Tool for Parental Tobacco Treatment in Hospitalized Children
- PMID: 27437049
- PMCID: PMC4941848
- DOI: 10.4338/aci-2015-12-ra-0169
Clinical Decision Support Tool for Parental Tobacco Treatment in Hospitalized Children
Abstract
Objectives: To create and evaluate the feasibility, acceptability, and usability of a clinical decision support (CDS) tool within the electronic health record (EHR) to help pediatricians provide smoking cessation counseling and treatment to parents of hospitalized children exposed to secondhand smoke (SHS).
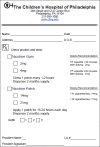
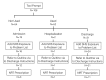
Methods: Mixed method study of first-year pediatric residents on one inpatient unit. Residents received training in smoking cessation counseling, nicotine replacement therapy (NRT) prescribing, and use of a CDS tool to aid in this process. The tool, which alerted when a patient was identified as exposed to SHS based on the history taken on admission or during a prior encounter, had the following capabilities: adding SHS exposure to the patient's problem list; referral to Free Quitline through discharge instructions; and linking to a printable NRT prescription form. We measured feasibility by EHR utilization data. We measured acceptability and usability of the tool by administering questionnaires to residents.
Results: From June-August 2015, the alert triggered for 106 patients, and the tool was used for 52 (49%) patients. 41 (39%) patients had SHS exposure added to the problem list, 34 (32%) parents were referred to the Quitline through discharge instructions, and 15 (14%) parents were prescribed NRT. 10 out of 15 (67%) eligible pediatricians used the tool. All clinicians surveyed (9 out of 10) found the tool acceptable and rated its usability good to excellent (average System Usability Scale score was 85 out of 100, 95% CI, 76-93).
Conclusions: A non-interruptive CDS tool to help residents provide smoking cessation counseling in the hospital was feasible, acceptable, and usable. Future work will investigate impacts on patient outcomes.
Keywords: Clinical decision support; secondhand smoke exposure; tobacco; usability.
Conflict of interest statement
The authors declare that they have no conflicts of interest in the research.
Figures
Similar articles
-
Clinical Decision Support Tool for Parental Tobacco Treatment in Primary Care.Pediatrics. 2016 May;137(5):e20154185. doi: 10.1542/peds.2015-4185. Epub 2016 Apr 14. Pediatrics. 2016. PMID: 27244817
-
Supporting Parents Living in Disadvantaged Areas of Edinburgh to Create a Smoke-Free Home Using Nicotine Replacement Therapy (NRT): A Two-Phase Qualitative Study.Int J Environ Res Public Health. 2020 Oct 7;17(19):7305. doi: 10.3390/ijerph17197305. Int J Environ Res Public Health. 2020. PMID: 33036327 Free PMC article.
-
Enhancing the electronic health record to increase counseling and quit-line referral for parents who smoke.Acad Pediatr. 2014 Sep-Oct;14(5):478-84. doi: 10.1016/j.acap.2014.03.017. Acad Pediatr. 2014. PMID: 25169159
-
Second hand smoke exposure in children: environmental factors, physiological effects, and interventions within pediatrics.Rev Environ Health. 2011;26(3):187-95. doi: 10.1515/reveh.2011.026. Rev Environ Health. 2011. PMID: 22206195 Review.
-
Managing tobacco use: the neglected cardiovascular disease risk factor.Eur Heart J. 2013 Nov;34(42):3259-67. doi: 10.1093/eurheartj/eht352. Epub 2013 Sep 7. Eur Heart J. 2013. PMID: 24014389 Review.
Cited by
-
Primary care provider adherence to an alert for intensification of diabetes blood pressure medications before and after the addition of a "chart closure" hard stop.J Am Med Inform Assoc. 2018 Sep 1;25(9):1167-1174. doi: 10.1093/jamia/ocy073. J Am Med Inform Assoc. 2018. PMID: 30060013 Free PMC article.
-
A Clinical Decision Support System for Motivational Messaging and Tobacco Cessation Treatment for Parents: Pilot Evaluation of Use and Acceptance.Appl Clin Inform. 2023 May;14(3):439-447. doi: 10.1055/a-2062-9627. Epub 2023 Mar 27. Appl Clin Inform. 2023. PMID: 36972687 Free PMC article.
-
Clinical decision support for tobacco screening and counseling parents of pediatric patients: A qualitative analysis of pediatric emergency department and urgent care professionals.Drug Alcohol Depend Rep. 2021 Dec 11;2:100019. doi: 10.1016/j.dadr.2021.100019. eCollection 2022 Mar. Drug Alcohol Depend Rep. 2021. PMID: 36845898 Free PMC article.
-
Home Smoke Exposure and Health-Related Quality of Life in Children with Acute Respiratory Illness.J Hosp Med. 2019 Apr;14(4):212-217. doi: 10.12788/jhm.3164. J Hosp Med. 2019. PMID: 30933671 Free PMC article.
-
An Electronic Health Record-Based Strategy to Address Child Tobacco Smoke Exposure.Am J Prev Med. 2018 Jan;54(1):64-71. doi: 10.1016/j.amepre.2017.08.011. Am J Prev Med. 2018. PMID: 29102458 Free PMC article.
References
-
- USDHHS: Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Washington, DC: US Government Printing Office; 2006.
-
- Jamal A, Homa DM, O’Connor E, Babb SD, Caraballo RS, Singh T, Hu SS, King BA. Current Cigarette Smoking Among Adults – United States, 2005–2014. MMWR Morb Mortal Wkly Rep 2015; 64(44):1233–1240. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

