Charged Nonclassical Antifolates with Activity Against Gram-Positive and Gram-Negative Pathogens
- PMID: 27437079
- PMCID: PMC4948012
- DOI: 10.1021/acsmedchemlett.6b00120
Charged Nonclassical Antifolates with Activity Against Gram-Positive and Gram-Negative Pathogens
Abstract
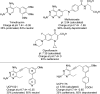
Although classical, negatively charged antifolates such as methotrexate possess high affinity for the dihydrofolate reductase (DHFR) enzyme, they are unable to penetrate the bacterial cell wall, rendering them poor antibacterial agents. Herein, we report a new class of charged propargyl-linked antifolates that capture some of the key contacts common to the classical antifolates while maintaining the ability to passively diffuse across the bacterial cell wall. Eight synthesized compounds exhibit extraordinary potency against Gram-positive S. aureus with limited toxicity against mammalian cells and good metabolic profile. High resolution crystal structures of two of the compounds reveal extensive interactions between the carboxylate and active site residues through a highly organized water network.
Keywords: Dihydrofolate reductase; Escherichia coli; MRSA; Staphylococcus aureus; antifolate; methotrexate; trimethoprim.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

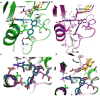
References
-
- Zhou W.; Scocchera E.; Wright D.; Anderson A. Antifolates as effective antimicrobial agents: new generations of trimethoprim analogs. MedChemComm 2013, 4, 908–915. 10.1039/c3md00104k. - DOI
-
- Li R.; Sirawaraporn R.; Chitnumsub P.; Sirawaraporn W.; Wooden J.; Athappilly F.; Turley S.; Hol W. Three-dimensional structure of M. tuberculosis dihydrofolate reductase reveals opportunities for the design of novel tuberculosis drugs. J. Mol. Biol. 2000, 295, 307–323. 10.1006/jmbi.1999.3328. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

