Identification of Novel Steroidal Androgen Receptor Degrading Agents Inspired by Galeterone 3β-Imidazole Carbamate
- PMID: 27437082
- PMCID: PMC4948004
- DOI: 10.1021/acsmedchemlett.6b00137
Identification of Novel Steroidal Androgen Receptor Degrading Agents Inspired by Galeterone 3β-Imidazole Carbamate
Abstract
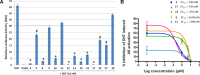
Degradation of all forms of androgen receptors (ARs) is emerging as an advantageous therapeutic paradigm for the effective treatment of prostate cancer. In continuation of our program to identify and develop improved efficacious novel small-molecule agents designed to disrupt AR signaling through enhanced AR degradation, we have designed, synthesized, and evaluated novel C-3 modified analogues of our phase 3 clinical agent, galeterone (5). Concerns of potential in vivo stability of our recently discovered more efficacious galeterone 3β-imidazole carbamate (6) led to the design and synthesis of new steroidal compounds. Two of the 11 compounds, 3β-pyridyl ether (8) and 3β-imidazole (17) with antiproliferative GI50 values of 3.24 and 2.54 μM against CWR22Rv1 prostate cancer cell, are 2.75- and 3.5-fold superior to 5. In addition, compounds 8 and 17 possess improved (∼4-fold) AR-V7 degrading activities. Importantly, these two compounds are expected to be metabolically stable, making them suitable for further development as new therapeutics against all forms of prostate cancer.
Keywords: Androgen receptor (AR); androgen receptor degrading agents (ARDAs); prostate cancer; splice variant AR (AR-V7).
Conflict of interest statement
The authors declare the following competing financial interest(s): V.C.O.N. is the lead inventor of galeterone and new analogues, patents, and technologies thereof owned by the University of Maryland, Baltimore, and licensed to Tokai Pharmaceuticals, Inc. A.K.K.-A. and P.P. are co-inventors of some related compounds. A patent application to protect these novel compounds has been filed.
Figures



Similar articles
-
Salinization Dramatically Enhance the Anti-Prostate Cancer Efficacies of AR/AR-V7 and Mnk1/2 Molecular Glue Degraders, Galeterone and VNPP433-3β Which Outperform Docetaxel and Enzalutamide in CRPC CWR22Rv1 Xenograft Mouse Model.Bioorg Chem. 2023 Oct;139:106700. doi: 10.1016/j.bioorg.2023.106700. Epub 2023 Jun 25. Bioorg Chem. 2023. PMID: 37392559 Free PMC article.
-
Androgen Receptor Modulation Optimized for Response-Splice Variant: A Phase 3, Randomized Trial of Galeterone Versus Enzalutamide in Androgen Receptor Splice Variant-7-expressing Metastatic Castration-resistant Prostate Cancer.Eur Urol. 2019 Dec;76(6):843-851. doi: 10.1016/j.eururo.2019.08.034. Epub 2019 Sep 18. Eur Urol. 2019. PMID: 31542304 Clinical Trial.
-
Systematic structure modifications of multitarget prostate cancer drug candidate galeterone to produce novel androgen receptor down-regulating agents as an approach to treatment of advanced prostate cancer.J Med Chem. 2013 Jun 27;56(12):4880-98. doi: 10.1021/jm400048v. Epub 2013 Jun 7. J Med Chem. 2013. PMID: 23713567 Free PMC article.
-
Galeterone for the treatment of advanced prostate cancer: the evidence to date.Drug Des Devel Ther. 2016 Jul 15;10:2289-97. doi: 10.2147/DDDT.S93941. eCollection 2016. Drug Des Devel Ther. 2016. PMID: 27486306 Free PMC article. Review.
-
Prognostic Value of Androgen Receptor Splice Variant 7 in the Treatment of Castration-resistant Prostate Cancer with Next generation Androgen Receptor Signal Inhibition: A Systematic Review and Meta-analysis.Eur Urol Focus. 2018 Jul;4(4):529-539. doi: 10.1016/j.euf.2017.01.004. Epub 2017 Jan 23. Eur Urol Focus. 2018. PMID: 28753843
Cited by
-
Discovery and development of steroidal enzyme inhibitors as anti-cancer drugs: state-of-the-art and future perspectives.J Enzyme Inhib Med Chem. 2025 Dec;40(1):2483818. doi: 10.1080/14756366.2025.2483818. Epub 2025 Apr 2. J Enzyme Inhib Med Chem. 2025. PMID: 40172115 Free PMC article. Review.
-
1,4-Substituted Triazoles as Nonsteroidal Anti-Androgens for Prostate Cancer Treatment.J Med Chem. 2017 Apr 13;60(7):3082-3093. doi: 10.1021/acs.jmedchem.7b00105. Epub 2017 Mar 20. J Med Chem. 2017. PMID: 28272894 Free PMC article.
-
Murine toxicology and pharmacokinetics of lead next generation galeterone analog, VNPP433-3β.Steroids. 2023 Apr;192:109184. doi: 10.1016/j.steroids.2023.109184. Epub 2023 Jan 24. Steroids. 2023. PMID: 36702363 Free PMC article.
-
Androgen receptor variant-driven prostate cancer II: advances in laboratory investigations.Prostate Cancer Prostatic Dis. 2020 Sep;23(3):381-397. doi: 10.1038/s41391-020-0217-3. Epub 2020 Mar 5. Prostate Cancer Prostatic Dis. 2020. PMID: 32139878 Free PMC article.
-
Novel AR/AR-V7 and Mnk1/2 Degrader, VNPP433-3β: Molecular Mechanisms of Action and Efficacy in AR-Overexpressing Castration Resistant Prostate Cancer In Vitro and In Vivo Models.Cells. 2022 Aug 30;11(17):2699. doi: 10.3390/cells11172699. Cells. 2022. PMID: 36078112 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

