Targeting Cancer Metabolism - Revisiting the Warburg Effects
- PMID: 27437085
- PMCID: PMC4946416
- DOI: 10.5487/TR.2016.32.3.177
Targeting Cancer Metabolism - Revisiting the Warburg Effects
Abstract
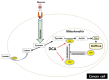
After more than half of century since the Warburg effect was described, this atypical metabolism has been standing true for almost every type of cancer, exhibiting higher glycolysis and lactate metabolism and defective mitochondrial ATP production. This phenomenon had attracted many scientists to the problem of elucidating the mechanism of, and reason for, this effect. Several models based on oncogenic studies have been proposed, such as the accumulation of mitochondrial gene mutations, the switch from oxidative phosphorylation respiration to glycolysis, the enhancement of lactate metabolism, and the alteration of glycolytic genes. Whether the Warburg phenomenon is the consequence of genetic dysregulation in cancer or the cause of cancer remains unknown. Moreover, the exact reasons and physiological values of this peculiar metabolism in cancer remain unclear. Although there are some pharmacological compounds, such as 2-deoxy-D-glucose, dichloroacetic acid, and 3-bromopyruvate, therapeutic strategies, including diet, have been developed based on targeting the Warburg effect. In this review, we will revisit the Warburg effect to determine how much scientists currently understand about this phenomenon and how we can treat the cancer based on targeting metabolism.
Keywords: Cancer metabolism; Energy metabolism; Mitochondria; Warburg effects.
Figures
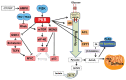
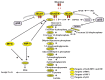
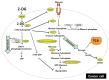
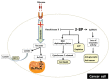
Similar articles
-
Revisiting the Warburg Effect: Diet-Based Strategies for Cancer Prevention.Biomed Res Int. 2020 Aug 4;2020:8105735. doi: 10.1155/2020/8105735. eCollection 2020. Biomed Res Int. 2020. PMID: 32802877 Free PMC article. Review.
-
The Warburg effect: molecular aspects and therapeutic possibilities.Mol Biol Rep. 2015 Apr;42(4):825-34. doi: 10.1007/s11033-014-3764-7. Mol Biol Rep. 2015. PMID: 25253100 Review.
-
The Warburg effect: essential part of metabolic reprogramming and central contributor to cancer progression.Int J Radiat Biol. 2019 Jul;95(7):912-919. doi: 10.1080/09553002.2019.1589653. Epub 2019 Mar 22. Int J Radiat Biol. 2019. PMID: 30822194 Review.
-
Warburg effect in Gynecologic cancers.J Obstet Gynaecol Res. 2019 Mar;45(3):542-548. doi: 10.1111/jog.13867. Epub 2018 Dec 3. J Obstet Gynaecol Res. 2019. PMID: 30511455 Review.
-
The HK2 Dependent "Warburg Effect" and Mitochondrial Oxidative Phosphorylation in Cancer: Targets for Effective Therapy with 3-Bromopyruvate.Molecules. 2016 Dec 15;21(12):1730. doi: 10.3390/molecules21121730. Molecules. 2016. PMID: 27983708 Free PMC article. Review.
Cited by
-
A Vaccine against Cancer: Can There Be a Possible Strategy to Face the Challenge? Possible Targets and Paradoxical Effects.Vaccines (Basel). 2023 Nov 8;11(11):1701. doi: 10.3390/vaccines11111701. Vaccines (Basel). 2023. PMID: 38006033 Free PMC article. Review.
-
Putrescine supplementation shifts macrophage L-arginine metabolism related-genes reducing Leishmania amazonensis infection.PLoS One. 2023 Mar 31;18(3):e0283696. doi: 10.1371/journal.pone.0283696. eCollection 2023. PLoS One. 2023. PMID: 37000792 Free PMC article.
-
Arrangement and symmetry of the fungal E3BP-containing core of the pyruvate dehydrogenase complex.Nat Commun. 2020 Sep 16;11(1):4667. doi: 10.1038/s41467-020-18401-z. Nat Commun. 2020. PMID: 32938938 Free PMC article.
-
Dysregulation of Glucose Metabolism by Oncogenes and Tumor Suppressors in Cancer Cells.Asian Pac J Cancer Prev. 2018 Sep 26;19(9):2377-2390. doi: 10.22034/APJCP.2018.19.9.2377. Asian Pac J Cancer Prev. 2018. PMID: 30255690 Free PMC article. Review.
-
Evaluation of a Novel Boron-Containing α-D-Mannopyranoside for BNCT.Cells. 2020 May 21;9(5):1277. doi: 10.3390/cells9051277. Cells. 2020. PMID: 32455737 Free PMC article.
References
-
- Warburg O. Notizen zur Entwickelungsphysiologie des Seeigeleies. Arch f d ges Physiol. 1915;160:324–332. doi: 10.1007/BF01680970. - DOI
-
- Warburg O. Versuche an überlebendem Carcinom-Gewebe (Methoden) Biochem Zeitschr. 1923;142:317–333.
-
- Warburg O. Verbesserte Methode zur Messung der Atmung und Glykolyse. Biochem Zeitschr. 1924;152:51–63.
-
- Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–270. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

