Propolis Inhibits Neurite Outgrowth in Differentiating SH-SY5Y Human Neuroblastoma Cells
- PMID: 27437091
- PMCID: PMC4946423
- DOI: 10.5487/TR.2016.32.3.239
Propolis Inhibits Neurite Outgrowth in Differentiating SH-SY5Y Human Neuroblastoma Cells
Erratum in
-
Erratum to "Propolis Inhibits Neurite Outgrowth in Differentiating SH-SY5Y Human Neuroblastoma Cells" [Toxicol. Res. 32 (2016) 239-243].Toxicol Res. 2016 Oct;32(4):359. doi: 10.5487/TR.2016.32.4.359. Epub 2016 Oct 30. Toxicol Res. 2016. PMID: 27818739 Free PMC article.
Abstract
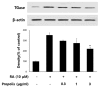
Propolis is a multicomponent, active, complex resinous substance collected by honeybees from a variety of plant sources. We have studied the effect of propolis on neurite outgrowth of SH-SY5Y human neuroblastoma cells induced to differentiate by all-trans-retinoic acid (RA). Propolis, at a concentration of 3 μg/mL, had no significant effect on the viability of differentiating SH-SY5Y cells. However, the neurite outgrowth of the differentiating SH-SY5Y cells treated with propolis (0.3~3 μg/mL) for 48 hr was significantly inhibited in a dose-dependent manner. Treatment of RA-stimulated differentiating SH-SY5Y cells with 0.3 to 3 μg/mL propolis resulted in decreased level of transglutaminase and 43-kDa growth-associated protein (GAP-43) in a dose-dependent manner. The results indicate that propolis is able to inhibit neurite outgrowth of differentiating SH-SY5Y cells.
Keywords: GAP-43; Neurite outgrowth; Propolis; SH-SY5Y neuroblastoma cell; TGase.
Figures



Similar articles
-
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) inhibits neurite outgrowth in differentiating human SH-SY5Y neuroblastoma cells.Toxicol Lett. 2009 Jul 24;188(2):153-6. doi: 10.1016/j.toxlet.2009.04.004. Epub 2009 Apr 10. Toxicol Lett. 2009. PMID: 19446249
-
Cadmium inhibits neurite outgrowth in differentiating human SH-SY5Y neuroblastoma cells.Int J Toxicol. 2014 Sep-Oct;33(5):412-8. doi: 10.1177/1091581814550338. Epub 2014 Sep 22. Int J Toxicol. 2014. PMID: 25249571
-
Protein kinase C-epsilon is implicated in neurite outgrowth in differentiating human neuroblastoma cells.Cell Growth Differ. 1996 Jun;7(6):775-85. Cell Growth Differ. 1996. PMID: 8780891
-
SH-SY5Y human neuroblastoma cell line: in vitro cell model of dopaminergic neurons in Parkinson's disease.Chin Med J (Engl). 2010 Apr 20;123(8):1086-92. Chin Med J (Engl). 2010. PMID: 20497720 Review.
-
Effect of Acrylamide and Mycotoxins in SH-SY5Y Cells: A Review.Toxins (Basel). 2024 Feb 6;16(2):87. doi: 10.3390/toxins16020087. Toxins (Basel). 2024. PMID: 38393165 Free PMC article. Review.
Cited by
-
Optimised techniques for high-throughput screening of differentiated SH-SY5Y cells and application for neurite outgrowth assays.Sci Rep. 2021 Dec 14;11(1):23935. doi: 10.1038/s41598-021-03442-1. Sci Rep. 2021. PMID: 34907283 Free PMC article.
-
Posidonia oceanica (L.) Delile Dampens Cell Migration of Human Neuroblastoma Cells.Mar Drugs. 2021 Oct 15;19(10):579. doi: 10.3390/md19100579. Mar Drugs. 2021. PMID: 34677478 Free PMC article.
-
Mechanism of Activating the Proprioceptive NT-3/TrkC Signalling Pathway by Reverse Intervention for the Anterior Cruciate Ligament-Hamstring Reflex Arc with Electroacupuncture.Biomed Res Int. 2018 Jan 18;2018:6348764. doi: 10.1155/2018/6348764. eCollection 2018. Biomed Res Int. 2018. PMID: 29581981 Free PMC article.
-
Caffeic Acid Phenethyl Ester (CAPE) Protects PC12 Cells from Cisplatin-Induced Neurotoxicity by Activating the NGF-Signaling Pathway.Neurotox Res. 2018 Jul;34(1):32-46. doi: 10.1007/s12640-017-9849-z. Epub 2017 Dec 19. Neurotox Res. 2018. PMID: 29260495
References
-
- Krol W, Czuba Z, Scheller S, Gabrys J, Grabiec S, Shani J. Anti-oxidant property of ethanolic extract of propolis (EEP) as evaluated by inhibiting the chemiluminescence oxidation of luminal. Biochem Int. 1990;21:593–597. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

