Probiotic isolates from unconventional sources: a review
- PMID: 27437119
- PMCID: PMC4949924
- DOI: 10.1186/s40781-016-0108-2
Probiotic isolates from unconventional sources: a review
Abstract
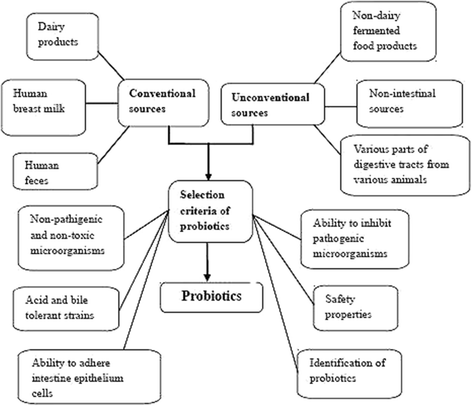
The use of probiotics for human and animal health is continuously increasing. The probiotics used in humans commonly come from dairy foods, whereas the sources of probiotics used in animals are often the animals' own digestive tracts. Increasingly, probiotics from sources other than milk products are being selected for use in people who are lactose intolerant. These sources are non-dairy fermented foods and beverages, non-dairy and non-fermented foods such as fresh fruits and vegetables, feces of breast-fed infants and human breast milk. The probiotics that are used in both humans and animals are selected in stages; after the initial isolation of the appropriate culture medium, the probiotics must meet important qualifications, including being non-pathogenic acid and bile-tolerant strains that possess the ability to act against pathogens in the gastrointestinal tract and the safety-enhancing property of not being able to transfer any antibiotic resistance genes to other bacteria. The final stages of selection involve the accurate identification of the probiotic species.
Keywords: Fermented food; Lactic acid bacteria; Probiotics; Unconventional sources.
Figures
References
-
- FAO/WHO. Guidelines for the evaluation of probiotics in food. London, Ontario, Canada: Food and Agriculture Organization of the United Nations and World Health Organization Working Group Report; 2002. Available at: ftp://ftp.fao.org/es/esn/food/wgreport2.pdf. Accessed 7 June 2016.
-
- Fuller R. Probiotic: the scientific basis. London: Chapman and Hall; 1992.
-
- Nousiainen J, Setala J. Lactic acid bacteria as animal probiotics. In: Salminen S, von WA, editors. Lactic acid bacteria. New York: Mercel Dekker Inc; 1998.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources