Monogenec Arrhythmic Syndromes: From Molecular and Genetic Aspects to Bedside
- PMID: 27437140
- PMCID: PMC4947989
Monogenec Arrhythmic Syndromes: From Molecular and Genetic Aspects to Bedside
Abstract
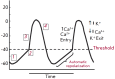
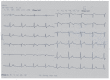
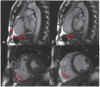
The abrupt cessation of effective cardiac function that is generally due to heart rhythm disorders can cause sudden and unexpected death at any age and is referred to as a syndrome called "sudden cardiac death" (SCD). Annually, about 400,000 cases of SCD occur in the United States alone. Less than 5% of the resuscitation techniques are effective. The prevalence of SCD in a population rises with age according to the prevalence of coronary artery disease, which is the most common cause of sudden cardiac arrest. However, there is a peak in SCD incidence for the age below 5 years, which is equal to 17 cases per 100,000 of the population. This peak is due to congenital monogenic arrhythmic canalopathies. Despite their relative rarity, these cases are obviously the most tragic. The immediate causes, or mechanisms, of SCD are comprehensive. Generally, it is arrhythmic death due to ventricular tachyarrythmias - sustained ventricular tachycardia (VT) or ventricular fibrillation (VF). Bradyarrhythmias and pulseless electrical activity account for no more than 40% of all registered cardiac arrests, and they are more often the outcome of the abovementioned arrhythmias. Our current understanding of the mechanisms responsible for SCD has emerged from decades of basic science investigation into the normal electrophysiology of the heart, the molecular physiology of cardiac ion channels, the fundamental cellular and tissue events associated with cardiac arrhythmias, and the molecular genetics of monogenic disorders of the heart rhythm (for example, the long QT syndrome). This review presents an overview of the molecular and genetic basis of SCD in the long QT syndrome, Brugada syndrome, short QT syndrome, catecholaminergic polymorphic ventricular tachycardia and idiopathic ventricular fibrillation, and arrhythmogenic right ventricular dysplasia, and sudden cardiac death prevention strategies by modern techniques (including implantable cardioverter-defibrillator).
Keywords: Brugada syndrome; arrhythmic right ventricular dysplasia; implantable cardioverter-defibrillator; long QT syndrome; monogenic canalopathy; sudden cardiac death.
Figures



References
-
- Revishvili A.Sh., Ardashev A.V., Bojcov S.A., Bockeria L.A., Golukhova E.Z., Davtyan K.V., Zenin S.A., Kuznetcov V.A., Kupcov V.V., Lebedev D.S. Clinical guidelines for electrophysiological studies, catheter ablation and implantable antiarrythmic devices usage. M.: MAKS Press; 2013 (in Russian) 2013. p. 596.
-
- Bockeria L.A., Revishvili A.Sh., Neminshchii I.M. Sudden cardiac death. M.: GEOTAR-Media; 2011. (in Russian) 2011. p. 272.
LinkOut - more resources
Full Text Sources
