Dynamic deformability of sickle red blood cells in microphysiological flow
- PMID: 27437432
- PMCID: PMC4947547
- DOI: 10.1142/S2339547816400045
Dynamic deformability of sickle red blood cells in microphysiological flow
Abstract
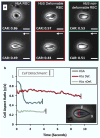
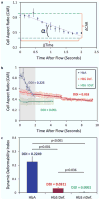
In sickle cell disease (SCD), hemoglobin molecules polymerize intracellularly and lead to a cascade of events resulting in decreased deformability and increased adhesion of red blood cells (RBCs). Decreased deformability and increased adhesion of sickle RBCs lead to blood vessel occlusion (vaso-occlusion) in SCD patients. Here, we present a microfluidic approach integrated with a cell dimensioning algorithm to analyze dynamic deformability of adhered RBC at the single-cell level in controlled microphysiological flow. We measured and compared dynamic deformability and adhesion of healthy hemoglobin A (HbA) and homozygous sickle hemoglobin (HbS) containing RBCs in blood samples obtained from 24 subjects. We introduce a new parameter to assess deformability of RBCs: the dynamic deformability index (DDI), which is defined as the time-dependent change of the cell's aspect ratio in response to fluid flow shear stress. Our results show that DDI of HbS-containing RBCs were significantly lower compared to that of HbA-containing RBCs. Moreover, we observed subpopulations of HbS containing RBCs in terms of their dynamic deformability characteristics: deformable and non-deformable RBCs. Then, we tested blood samples from SCD patients and analyzed RBC adhesion and deformability at physiological and above physiological flow shear stresses. We observed significantly greater number of adhered non-deformable sickle RBCs than deformable sickle RBCs at flow shear stresses well above the physiological range, suggesting an interplay between dynamic deformability and increased adhesion of RBCs in vaso-occlusive events.
Keywords: Biomechanics; Cell Adhesion; Cell Mechanics; Dynamic Cell Deformation; Microfluidics; Red Blood Cell; Sickle Cell Disease.
Conflict of interest statement
The authors declare no competing financial interests. Y.A., J.A.L. and U.A.G. filed a patent application pertaining to the results presented in this paper: Patent Cooperation Treaty Application (PCT/US2015/042907): “Biochips to Diagnose Hemoglobin Disorders and Monitor Blood Cells”.
Figures



References
-
- Juhan I, et al. Abnormalities of erythrocyte deformability and platelet aggregation in insulin-dependent diabetics corrected by insulin in vivo and in vitro. Lancet. 1982;1:535–537. - PubMed
-
- Diamantopoulos EJ, et al. Impaired erythrocyte deformability precedes vascular changes in experimental diabetes mellitus. Horm Metab Res. 2004;36:142–147. - PubMed
-
- Glenister FK, Coppel RL, Cowman AF, Mohandas N, Cooke BM. Contribution of parasite proteins to altered mechanical properties of malaria-infected red blood cells. Blood. 2002;99:1060–1063. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
