Data on how several physiological parameters of stored red blood cells are similar in glucose 6-phosphate dehydrogenase deficient and sufficient donors
- PMID: 27437434
- PMCID: PMC4939423
- DOI: 10.1016/j.dib.2016.06.018
Data on how several physiological parameters of stored red blood cells are similar in glucose 6-phosphate dehydrogenase deficient and sufficient donors
Abstract
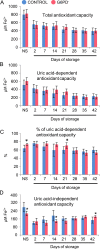
This article contains data on the variation in several physiological parameters of red blood cells (RBCs) donated by eligible glucose-6-phosphate dehydrogenase (G6PD) deficient donors during storage in standard blood bank conditions compared to control, G6PD sufficient (G6PD(+)) cells. Intracellular reactive oxygen species (ROS) generation, cell fragility and membrane exovesiculation were measured in RBCs throughout the storage period, with or without stimulation by oxidants, supplementation of N-acetylcysteine and energy depletion, following incubation of stored cells for 24 h at 37 °C. Apart from cell characteristics, the total or uric acid-dependent antioxidant capacity of the supernatant in addition to extracellular potassium concentration was determined in RBC units. Finally, procoagulant activity and protein carbonylation levels were measured in the microparticles population. Further information can be found in "Glucose 6-phosphate dehydrogenase deficient subjects may be better "storers" than donors of red blood cells" [1].
Keywords: AnnV, annexin V; CPD, citrate-phosphate-dextrose; Cell fragility; FRAP, ferric reducing antioxidant power; FSC, forward scatter; G6PD deficiency; G6PD, glucose-6-phosphate dehydrogenase; G6PD−, G6PD deficiency; Hb, hemoglobin; Hct, hematocrit; K+, potassium; MCF, mean corpuscular fragility; MFI, mechanical fragility index; MP, micoparticles, microvesicles; MPPA, microparticles pro-coagulant activity; Microparticles; NAC, N-acetylcysteine; NS, non-stored; Oxidative stress; PBS, phosphate buffer saline; PCI, protein carbonylation index; PS, phosphatidylserine; RBC, red blood cell; RFU, relative fluorescence units; ROS, reactive oxygen species; Red blood cell storage lesion; SAGM, saline-adenine-glucose-mannitol; SSC, side scatter; TAC, total antioxidant capacity; UA-dep AC, uric acid dependent antioxidant capacity; UA-ind AC, uric acid independent antioxidant capacity; tBHP, tert-Butyl hydroperoxide.
Figures




References
-
- Tzounakas V.L., Kriebardis A.G., Georgatzakou H.T., Foudoulaki-Paparizos L.E., Dzieciatkowska M., Wither M.J., Nemkov T., Hansen K.C., Papassideri I.S., D׳Alessandro A., Antonelou M.H. Glucose 6-phosphate dehydrogenase deficient subjects may be better "storers" than donors of red blood cells. Free Radic. Biol. Med. 2016;96:152–165. - PubMed
-
- Antonelou M.H., Tzounakas V.L., Velentzas A.D., Stamoulis K.E., Kriebardis A.G., Papassideri I.S. Effects of pre-storage leukoreduction on stored red blood cells signaling: a time-course evaluation from shape to proteome. J Proteom. 2012;76:220–238. Spec No. - PubMed
-
- Benzie I.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of "antioxidant power": the FRAP assay. Anal. Biochem. 1996;239:70–76. - PubMed
-
- Harboe M. A method for determination of hemoglobin in plasma by near-ultraviolet spectrophotometry. Scand. J Clin. Lab. Investig. 1959;11:66–70. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

