Structural basis of Smoothened regulation by its extracellular domains
- PMID: 27437577
- PMCID: PMC4970916
- DOI: 10.1038/nature18934
Structural basis of Smoothened regulation by its extracellular domains
Abstract
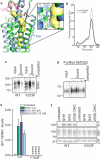
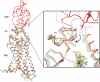
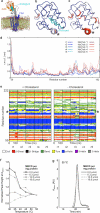
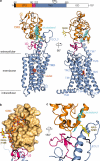
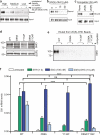
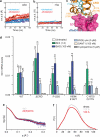
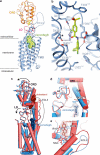
Developmental signals of the Hedgehog (Hh) and Wnt families are transduced across the membrane by Frizzledclass G-protein-coupled receptors (GPCRs) composed of both a heptahelical transmembrane domain (TMD) and an extracellular cysteine-rich domain (CRD). How the large extracellular domains of GPCRs regulate signalling by the TMD is unknown. We present crystal structures of the Hh signal transducer and oncoprotein Smoothened, a GPCR that contains two distinct ligand-binding sites: one in its TMD and one in the CRD. The CRD is stacked a top the TMD, separated by an intervening wedge-like linker domain. Structure-guided mutations show that the interface between the CRD, linker domain and TMD stabilizes the inactive state of Smoothened. Unexpectedly, we find a cholesterol molecule bound to Smoothened in the CRD binding site. Mutations predicted to prevent cholesterol binding impair the ability of Smoothened to transmit native Hh signals. Binding of a clinically used antagonist, vismodegib, to the TMD induces a conformational change that is propagated to the CRD, resulting in loss of cholesterol from the CRD-linker domain-TMD interface. Our results clarify the structural mechanism by which the activity of a GPCR is controlled by ligand-regulated interactions between its extracellular and transmembrane domains.
Figures


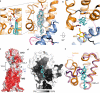
References
-
- Sharpe HJ, Wang W, Hannoush RN, de Sauvage FJ. Regulation of the oncoprotein Smoothened by small molecules. Nature chemical biology. 2015;11:246–255. doi:10.1038/nchembio.1776. - PubMed
-
- Taipale J, et al. Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature. 2000;406:1005–1009. doi:10.1038/35023008. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 102890/Z/13/Z/WT_/Wellcome Trust/United Kingdom
- R35 GM118082/GM/NIGMS NIH HHS/United States
- GM106078/GM/NIGMS NIH HHS/United States
- 14414/CRUK_/Cancer Research UK/United Kingdom
- C20724/A14414/CRUK_/Cancer Research UK/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- 092970/Z/10/Z/WT_/Wellcome Trust/United Kingdom
- R01 GM106078/GM/NIGMS NIH HHS/United States
- T32 GM007276/GM/NIGMS NIH HHS/United States
- HL067773/HL/NHLBI NIH HHS/United States
- MRC_/Medical Research Council/United Kingdom
- 090532/Z/09/Z/WT_/Wellcome Trust/United Kingdom
- R01 HL067773/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

