Defining a Research Agenda for Patient-Reported Outcomes in Surgery: Using a Delphi Survey of Stakeholders
- PMID: 27437666
- PMCID: PMC5216456
- DOI: 10.1001/jamasurg.2016.1640
Defining a Research Agenda for Patient-Reported Outcomes in Surgery: Using a Delphi Survey of Stakeholders
Abstract
Importance: Identifying timely and important research questions using relevant patient-reported outcomes (PROs) in surgery remains paramount in the current medical climate. The inaugural Patient-Reported Outcomes in Surgery (PROS) Conference brought together stakeholders in PROs research in surgery with the aim of creating a research agenda to help determine future directions and advance cross-disciplinary collaboration.
Objective: To create a research agenda to help determine future directions and advance cross-disciplinary collaboration on the use of PROs in surgery.
Design, setting, and participants: An iterative web-based interface was used to create a conference-based, modified Delphi survey for registrants at the PROS Conference (January 29-30, 2015), including surgeons, PRO researchers, payers, and other stakeholders. In round 1, research items were generated from qualitative review of responses to open-ended prompts. In round 2, items were ranked using a 5-point Likert scale; attendees were also asked to submit any new items. In round 3, the top 30 items and newly submitted items were redistributed for final ranking using a 3-point Likert scale. The top 20 items by mean rating were selected for the research agenda.
Main outcomes and measures: An expert-generated research agenda on PROs in surgery.
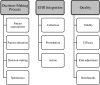
Results: Of the 143 people registered for the conference, 137 provided valid email addresses. There was a wide range of attendees, with the 3 most common groups being plastic surgeons (28 [19.6%]), general surgeons (19 [13.3%]), and researchers (25 [17.5%]). In round 1, participants submitted 459 items, which were reduced through qualitative review to 53 distinct items across 7 themes of PROs research. A research agenda was formulated after 2 successive rounds of ranking. The research agenda identified 3 themes important for future PROs research in surgery: (1) PROs in the decision-making process, (2) integrating PROs into the electronic health record, and (3) measuring quality in surgery with PROs.
Conclusions and relevance: The PROS Conference research agenda was created using a modified Delphi survey of stakeholders that will help researchers, surgeons, and funders identify crucial areas of future PROs research in surgery.
Figures
Comment in
-
How Can a Valid Research Agenda for Patient-Reported Outcomes Be Defined Without Patient Input?JAMA Surg. 2016 Oct 1;151(10):936-937. doi: 10.1001/jamasurg.2016.1695. JAMA Surg. 2016. PMID: 27438653 No abstract available.
Similar articles
-
SAGES research agenda in gastrointestinal and endoscopic surgery: updated results of a Delphi study.Surg Endosc. 2014 Oct;28(10):2763-71. doi: 10.1007/s00464-014-3535-5. Epub 2014 May 2. Surg Endosc. 2014. PMID: 24789129
-
Defining a Research Agenda for Layperson Prehospital Hemorrhage Control: A Consensus Statement.JAMA Netw Open. 2020 Jul 1;3(7):e209393. doi: 10.1001/jamanetworkopen.2020.9393. JAMA Netw Open. 2020. PMID: 32663307
-
Development of ASMBS research agenda for bariatric surgery using the Delphi methodology.Surg Obes Relat Dis. 2019 Sep;15(9):1563-1569. doi: 10.1016/j.soard.2019.06.043. Epub 2019 Jul 8. Surg Obes Relat Dis. 2019. PMID: 31466874
-
Ethical Considerations for the Inclusion of Patient-Reported Outcomes in Clinical Research: The PRO Ethics Guidelines.JAMA. 2022 May 17;327(19):1910-1919. doi: 10.1001/jama.2022.6421. JAMA. 2022. PMID: 35579638
-
Identifying a Core Domain Set to Assess Psoriasis in Clinical Trials.JAMA Dermatol. 2018 Oct 1;154(10):1137-1144. doi: 10.1001/jamadermatol.2018.1165. JAMA Dermatol. 2018. PMID: 29874367 Free PMC article.
Cited by
-
Outcomes in Deep Inferior Epigastric Perforator Flap and Implant-Based Reconstruction: Does Age Really Matter?Cancer Control. 2018 Jan-Mar;25(1):1073274817744603. doi: 10.1177/1073274817744603. Cancer Control. 2018. PMID: 29325422 Free PMC article. Review.
-
Comparison of early patient-reported outcomes between uniportal thoracoscopic segmentectomy and wedge resection for peripheral small-sized non-small-cell lung cancer.J Cardiothorac Surg. 2024 Apr 15;19(1):215. doi: 10.1186/s13019-024-02635-9. J Cardiothorac Surg. 2024. PMID: 38622650 Free PMC article.
-
The Critical Portions of Carpal Tunnel Release, Ulnar Nerve Transposition, and Open Reduction and Internal Fixation of the Distal Part of the Radius.J Bone Joint Surg Am. 2018 Dec 5;100(23):e148. doi: 10.2106/JBJS.17.00654. J Bone Joint Surg Am. 2018. PMID: 30516635 Free PMC article.
-
Ethical Orientation and Research Misconduct Among Business Researchers Under the Condition of Autonomy and Competition.J Bus Ethics. 2023;183(2):619-636. doi: 10.1007/s10551-022-05043-y. Epub 2022 Jan 29. J Bus Ethics. 2023. PMID: 35125566 Free PMC article.
-
Reliability and validity of the Turkish version of the New Cleveland Clinic Colorectal Cancer Quality of Life Questionnaire.Int J Colorectal Dis. 2023 Dec 27;39(1):10. doi: 10.1007/s00384-023-04572-w. Int J Colorectal Dis. 2023. PMID: 38150157
References
-
- Food and Drug Administration (US) Guidance for Industry. Patient Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. Fed Regist. 2009;74(235):65132–65133. - PubMed
-
- Möller E, Weidenhielm L, Werner S. Outcome and knee-related quality of life after anterior cruciate ligament reconstruction: a long-term follow-up. Knee Surg Sports Traumatol Arthrosc. 2009;17(7):786–794. - PubMed
-
- Pusic AL, Klassen AF, Scott AM, Klok JA, Cordeiro PG, Cano SJ. Development of a new patient-reported outcome measure for breast surgery: the BREAST-Q. Plast Reconstr Surg. 2009;124(2):345–353. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous