FBN1: The disease-causing gene for Marfan syndrome and other genetic disorders
- PMID: 27437668
- PMCID: PMC6639799
- DOI: 10.1016/j.gene.2016.07.033
FBN1: The disease-causing gene for Marfan syndrome and other genetic disorders
Abstract
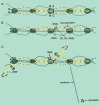
FBN1 encodes the gene for fibrillin-1, a structural macromolecule that polymerizes into microfibrils. Fibrillin microfibrils are morphologically distinctive fibrils, present in all connective tissues and assembled into tissue-specific architectural frameworks. FBN1 is the causative gene for Marfan syndrome, an inherited disorder of connective tissue whose major features include tall stature and arachnodactyly, ectopia lentis, and thoracic aortic aneurysm and dissection. More than one thousand individual mutations in FBN1 are associated with Marfan syndrome, making genotype-phenotype correlations difficult. Moreover, mutations in specific regions of FBN1 can result in the opposite features of short stature and brachydactyly characteristic of Weill-Marchesani syndrome and other acromelic dysplasias. How can mutations in one molecule result in disparate clinical syndromes? Current concepts of the fibrillinopathies require an appreciation of tissue-specific fibrillin microfibril microenvironments and the collaborative relationship between the structures of fibrillin microfibril networks and biological functions such as regulation of growth factor signaling.
Keywords: FBN1; Fibrillin; Fibrillinopathies; Marfan syndrome; Microenvironment; Thoracic aortic aneurysm; Weill–Marchesani syndrome.
Copyright © 2016 Elsevier B.V. All rights reserved.
Figures



References
-
- Gibson MA, Hughes JL, Fanning JC, Cleary EG. The major antigen of elastin-associated microfibrils is a 31-kDa glycoprotein. J Biol Chem. 1986;261:11429–11436. - PubMed
-
- Reinhardt DP, Sasaki T, Dzamba BJ, Keene DR, Chu ML, Gӧhring W, Timpl R, Sakai LY. Fibrillin-1 and fibulin-2 interact and are colocalized in some tissues. J Biol Chem. 1996;271:19489–19496. - PubMed
-
- El-Hallous E, Sasaki T, Hubmacher D, Getie M, Tiedemann K, Brinckmann J, Bӓtge B, Davis EC, Reinhardt DP. Fibrillin-1 interactions with fibulins depend on the first hybrid domain and provide an adaptor function to tropoelastin. J Biol Chem. 2007;282:8935–8946. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

