Progress and perspective of TBX6 gene in congenital vertebral malformations
- PMID: 27437870
- PMCID: PMC5302999
- DOI: 10.18632/oncotarget.10619
Progress and perspective of TBX6 gene in congenital vertebral malformations
Abstract
Congenital vertebral malformation is a series of significant health problems affecting a large number of populations. It may present as an isolated condition or as a part of an underlying syndromes occurring with other malformations and/or clinical features. Disruption of the genesis of paraxial mesoderm, somites or axial bones can result in spinal deformity. In the course of somitogenesis, the segmentation clock and the wavefront are the leading factors during the entire process in which TBX6 gene plays an important role. TBX6 is a member of the T-box gene family, and its important pathogenicity in spinal deformity has been confirmed. Several TBX6 gene variants and novel pathogenic mechanisms have been recently revealed, and will likely have significant impact in understanding the genetic basis for CVM. In this review, we describe the role which TBX6 plays during human spine development including its interaction with other key elements during the process of somitogenesis. We then systematically review the association between TBX6 gene variants and CVM associated phenotypes, highlighting an important and emerging role for TBX6 and human malformations.
Keywords: TBX6; congenital scoliosis; congenital vertebral malformation; somitogenesis; vertebrate segmentation.
Conflict of interest statement
The authors declare no potential conflicts of interests.
Figures
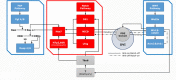
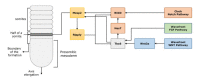
References
-
- Tawk RG, Ondra SL, Jorge AM, Morrison T, Ganju A. Hypersegmentation, Klippel-Feil syndrome, and hemivertebra in a scoliotic patient. J Am Coll Surg. 2002;195(4):570–571. - PubMed
-
- Giampietro PF, Dunwoodie SL, Kusumi K, Pourquie O, Tassy O, Offiah AC, Cornier AS, Alman BA, Blank RD, Raggio CL, Glurich I, Turnpenny PD. Progress in the understanding of the genetic etiology of vertebral segmentation disorders in humans. Ann N Y Acad Sci. 2009;1151:38–67. - PubMed
-
- Brand MC. Examination of the newborn with congenital scoliosis: focus on the physical. Adv Neonatal Care. 2008;8(5):265–273. 274–275. - PubMed
-
- Dayem-Quere M, Giuliano F, Wagner-Mahler K, Massol C, Crouzet-Ozenda L, Lambert JC, Karmous-Benailly H. Delineation of a region responsible for panhypopituitarism in 20p11.2. Am J Med Genet A. 2013;161A(7):1547–1554. - PubMed
-
- Bulman MP, Kusumi K, Frayling TM, McKeown C, Garrett C, Lander ES, Krumlauf R, Hattersley AT, Ellard S, Turnpenny PD. Mutations in the human delta homologue, DLL3, cause axial skeletal defects in spondylocostal dysostosis. Nat Genet. 2000;24(4):438–441. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

