The Role of Reactive Oxygen Species in Antibiotic-Induced Cell Death in Burkholderia cepacia Complex Bacteria
- PMID: 27438061
- PMCID: PMC4954720
- DOI: 10.1371/journal.pone.0159837
The Role of Reactive Oxygen Species in Antibiotic-Induced Cell Death in Burkholderia cepacia Complex Bacteria
Abstract
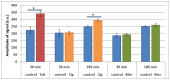
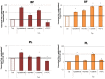
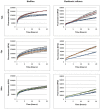
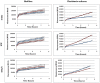
It was recently proposed that bactericidal antibiotics, besides through specific drug-target interactions, kill bacteria by a common mechanism involving the production of reactive oxygen species (ROS). However, this mechanism involving the production of hydroxyl radicals has become the subject of a lot of debate. Since the contribution of ROS to antibiotic mediated killing most likely depends on the conditions, differences in experimental procedures are expected to be at the basis of the conflicting results. In the present study different methods (ROS specific stainings, gene-expression analyses, electron paramagnetic resonance, genetic and phenotypic experiments, detection of protein carbonylation and DNA oxidation) to measure the production of ROS upon antibiotic treatment in Burkholderia cepacia complex (Bcc) bacteria were compared. Different classes of antibiotics (tobramycin, ciprofloxacin, meropenem) were included, and both planktonic and biofilm cultures were studied. Our results indicate that some of the methods investigated were not sensitive enough to measure antibiotic induced production of ROS, including the spectrophotometric detection of protein carbonylation. Secondly, other methods were found to be useful only in specific conditions. For example, an increase in the expression of OxyR was measured in Burkholderia cenocepacia K56-2 after treatment with ciprofloxacin or meropenem (both in biofilms and planktonic cultures) but not after treatment with tobramycin. In addition results vary with the experimental conditions and the species tested. Nevertheless our data strongly suggest that ROS contribute to antibiotic mediated killing in Bcc species and that enhancing ROS production or interfering with the protection against ROS may form a novel strategy to improve antibiotic treatment.
Conflict of interest statement
Figures


Similar articles
-
In vitro activity of ceftazidime, ciprofloxacin, meropenem, minocycline, tobramycin and trimethoprim/sulfamethoxazole against planktonic and sessile Burkholderia cepacia complex bacteria.J Antimicrob Chemother. 2009 Oct;64(4):801-9. doi: 10.1093/jac/dkp253. Epub 2009 Jul 23. J Antimicrob Chemother. 2009. PMID: 19633000
-
Investigating the role of matrix components in protection of Burkholderia cepacia complex biofilms against tobramycin.J Cyst Fibros. 2014 Jan;13(1):56-62. doi: 10.1016/j.jcf.2013.07.004. Epub 2013 Aug 8. J Cyst Fibros. 2014. PMID: 23932109
-
Biofilm-grown Burkholderia cepacia complex cells survive antibiotic treatment by avoiding production of reactive oxygen species.PLoS One. 2013;8(3):e58943. doi: 10.1371/journal.pone.0058943. Epub 2013 Mar 13. PLoS One. 2013. PMID: 23516582 Free PMC article.
-
Social interactions in the Burkholderia cepacia complex: biofilms and quorum sensing.Future Microbiol. 2010 Jul;5(7):1087-99. doi: 10.2217/fmb.10.68. Future Microbiol. 2010. PMID: 20632807 Review.
-
[Advances in virulence determinants in Burkholderia cepacia complex--a review].Wei Sheng Wu Xue Bao. 2014 May 4;54(5):487-97. Wei Sheng Wu Xue Bao. 2014. PMID: 25199247 Review. Chinese.
Cited by
-
Caffeic Acid Esters Are Effective Bactericidal Compounds Against Paenibacilluslarvae by Altering Intracellular Oxidant and Antioxidant Levels.Biomolecules. 2019 Jul 27;9(8):312. doi: 10.3390/biom9080312. Biomolecules. 2019. PMID: 31357646 Free PMC article.
-
Effect of Size and Concentration of Copper Nanoparticles on the Antimicrobial Activity in Escherichia coli through Multiple Mechanisms.Nanomaterials (Basel). 2022 Oct 22;12(21):3715. doi: 10.3390/nano12213715. Nanomaterials (Basel). 2022. PMID: 36364491 Free PMC article.
-
Profiling cell envelope-antibiotic interactions reveals vulnerabilities to β-lactams in a multidrug-resistant bacterium.Nat Commun. 2023 Aug 9;14(1):4815. doi: 10.1038/s41467-023-40494-5. Nat Commun. 2023. PMID: 37558695 Free PMC article.
-
Mutations in the Staphylococcus aureus Global Regulator CodY Confer Tolerance to an Interspecies Redox-Active Antimicrobial.bioRxiv [Preprint]. 2024 Jul 3:2024.07.02.601769. doi: 10.1101/2024.07.02.601769. bioRxiv. 2024. Update in: PLoS Genet. 2025 Mar 07;21(3):e1011610. doi: 10.1371/journal.pgen.1011610. PMID: 39040146 Free PMC article. Updated. Preprint.
-
Photo-Responsive Ascorbic Acid-Modified Ag2S-ZnS Heteronanostructure Dropping pH to Trigger Synergistic Antibacterial and Bohr Effects for Accelerating Infected Wound Healing.ACS Appl Mater Interfaces. 2024 Mar 6;16(9):12018-12032. doi: 10.1021/acsami.3c17424. Epub 2024 Feb 23. ACS Appl Mater Interfaces. 2024. PMID: 38394675 Free PMC article.
References
-
- Imlay JA, Fridovich I. Superoxide production by respiring membranes of Escherichia coli. Free radic Res Commun. 1991;12–13 Pt 1:59–66. - PubMed
-
- Kohanski MA, Dwyer DJ, Hayete B, Lawrence CA, Collins JJ. A common mechanism of cellular death induced by bactericidal antibiotics. Cell. 2007;130(5):797–810. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

