Subretinal mononuclear phagocytes induce cone segment loss via IL-1β
- PMID: 27438413
- PMCID: PMC4969036
- DOI: 10.7554/eLife.16490
Subretinal mononuclear phagocytes induce cone segment loss via IL-1β
Abstract
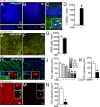
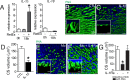
Photo-transduction in cone segments (CS) is crucial for high acuity daytime vision. For ill-defined reasons, CS degenerate in retinitis pigmentosa (RP) and in the transitional zone (TZ) of atrophic zones (AZ), which characterize geographic atrophy (GA). Our experiments confirm the loss of cone segments (CS) in the TZ of patients with GA and show their association with subretinal CD14(+)mononuclear phagocyte (MP) infiltration that is also reported in RP. Using human and mouse MPs in vitro and inflammation-prone Cx3cr1(GFP/GFP) mice in vivo, we demonstrate that MP-derived IL-1β leads to severe CS degeneration. Our results strongly suggest that subretinal MP accumulation participates in the observed pathological photoreceptor changes in these diseases. Inhibiting subretinal MP accumulation or Il-1β might protect the CS and help preserve high acuity daytime vision in conditions characterized by subretinal inflammation, such as AMD and RP.
Keywords: age-related macular degeneration; human; human biology; interleukin; macrophages; medicine; mouse; neuroscience; outer segments; retinitis pigmentosa; subretinal space.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures




Similar articles
-
CD36 Deficiency Inhibits Retinal Inflammation and Retinal Degeneration in Cx3cr1 Knockout Mice.Front Immunol. 2020 Jan 8;10:3032. doi: 10.3389/fimmu.2019.03032. eCollection 2019. Front Immunol. 2020. PMID: 31969887 Free PMC article.
-
Upregulation of P2RX7 in Cx3cr1-Deficient Mononuclear Phagocytes Leads to Increased Interleukin-1β Secretion and Photoreceptor Neurodegeneration.J Neurosci. 2015 May 6;35(18):6987-96. doi: 10.1523/JNEUROSCI.3955-14.2015. J Neurosci. 2015. PMID: 25948251 Free PMC article.
-
Apolipoprotein E promotes subretinal mononuclear phagocyte survival and chronic inflammation in age-related macular degeneration.EMBO Mol Med. 2015 Feb;7(2):211-26. doi: 10.15252/emmm.201404524. EMBO Mol Med. 2015. PMID: 25604058 Free PMC article.
-
On phagocytes and macular degeneration.Prog Retin Eye Res. 2017 Nov;61:98-128. doi: 10.1016/j.preteyeres.2017.06.002. Epub 2017 Jun 7. Prog Retin Eye Res. 2017. PMID: 28602950 Review.
-
Defective cone photoreceptor cytoskeleton, alignment, feedback, and energetics can lead to energy depletion in macular degeneration.Prog Retin Eye Res. 2004 Sep;23(5):495-522. doi: 10.1016/j.preteyeres.2004.04.005. Prog Retin Eye Res. 2004. PMID: 15302348 Review.
Cited by
-
HISTOLOGY OF GEOGRAPHIC ATROPHY SECONDARY TO AGE-RELATED MACULAR DEGENERATION: A Multilayer Approach.Retina. 2018 Oct;38(10):1937-1953. doi: 10.1097/IAE.0000000000002182. Retina. 2018. PMID: 29746415 Free PMC article.
-
Retinal Structure and Microcirculation Alterations in Patients With Papillary Thyroid Carcinoma: An Optical Coherence Tomography and Angiography Study.Invest Ophthalmol Vis Sci. 2025 Apr 1;66(4):73. doi: 10.1167/iovs.66.4.73. Invest Ophthalmol Vis Sci. 2025. PMID: 40272368 Free PMC article.
-
NLRP3 Activation With Bisphosphonate Use and the Risk of Incident Age-Related Macular Degeneration.Invest Ophthalmol Vis Sci. 2025 Mar 3;66(3):32. doi: 10.1167/iovs.66.3.32. Invest Ophthalmol Vis Sci. 2025. PMID: 40094655 Free PMC article.
-
Exploring the pathogenesis of age-related macular degeneration: A review of the interplay between retinal pigment epithelium dysfunction and the innate immune system.Front Neurosci. 2022 Nov 3;16:1009599. doi: 10.3389/fnins.2022.1009599. eCollection 2022. Front Neurosci. 2022. PMID: 36408381 Free PMC article. Review.
-
Chronic exposure to tumor necrosis factor alpha induces retinal pigment epithelium cell dedifferentiation.J Neuroinflammation. 2018 Mar 16;15(1):85. doi: 10.1186/s12974-018-1106-8. J Neuroinflammation. 2018. PMID: 29548329 Free PMC article.
References
-
- Aït-Ali N, Fridlich R, Millet-Puel G, Clérin E, Delalande F, Jaillard C, Blond F, Perrocheau L, Reichman S, Byrne LC, Olivier-Bandini A, Bellalou J, Moyse E, Bouillaud F, Nicol X, Dalkara D, van Dorsselaer A, Sahel JA, Léveillard T. Rod-derived cone viability factor promotes cone survival by stimulating aerobic glycolysis. Cell. 2015;161:817–832. doi: 10.1016/j.cell.2015.03.023. - DOI - PubMed
-
- Blanks JC, Johnson LV. Specific binding of peanut lectin to a class of retinal photoreceptor cells. A species comparison. Investigative Ophthalmology & Visual Science. 1984;25:546–557. - PubMed
-
- Combadière C, Feumi C, Raoul W, Keller N, Rodéro M, Pézard A, Lavalette S, Houssier M, Jonet L, Picard E, Debré P, Sirinyan M, Deterre P, Ferroukhi T, Cohen S-Y, Chauvaud D, Jeanny J-C, Chemtob S, Behar-Cohen F, Sennlaub F. CX3CR1-dependent subretinal microglia cell accumulation is associated with cardinal features of age-related macular degeneration. Journal of Clinical Investigation. 2007;117:2920–2928. doi: 10.1172/JCI31692. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

