Chronic Treatment with Isoniazid Causes Protoporphyrin IX Accumulation in Mouse Liver
- PMID: 27438535
- PMCID: PMC5316289
- DOI: 10.1021/acs.chemrestox.6b00121
Chronic Treatment with Isoniazid Causes Protoporphyrin IX Accumulation in Mouse Liver
Abstract
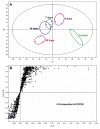
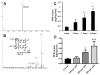
Isoniazid (INH) can cause hepatotoxicity. In addition, INH is contraindicated in patients suffering from porphyrias. Our metabolomic analysis revealed that chronic treatment with INH in mice causes a hepatic accumulation of protoporphyrin IX (PPIX). PPIX is an intermediate in the heme biosynthesis pathway, and it is also known as a hepatotoxin. We further found that INH induces delta-aminolevulinate synthase 1 (ALAS1), the rate-limiting enzyme in heme biosynthesis. We also found that INH downregulates ferrochelatase (FECH), the enzyme that converts PPIX to heme. In summary, this study illustrated that chronic treatment with INH causes PPIX accumulation in mouse liver in part through ALAS1 induction and FECH downregulation. This study also highlights that drugs can disrupt the metabolic pathways of endobiotics and increase the risk of liver damage.
Conflict of interest statement
The authors declare to have no conflict of interest.
Figures



Similar articles
-
Results of a pilot study of isoniazid in patients with erythropoietic protoporphyria.Mol Genet Metab. 2019 Nov;128(3):309-313. doi: 10.1016/j.ymgme.2019.07.017. Epub 2019 Jul 31. Mol Genet Metab. 2019. PMID: 31395332 Free PMC article.
-
Involvement of protoporphyrin IX accumulation in the pathogenesis of isoniazid/rifampicin-induced liver injury: the prevention of curcumin.Xenobiotica. 2017 Feb;47(2):154-163. doi: 10.3109/00498254.2016.1160159. Xenobiotica. 2017. PMID: 28118809
-
The Isoniazid Metabolites Hydrazine and Pyridoxal Isonicotinoyl Hydrazone Modulate Heme Biosynthesis.Toxicol Sci. 2019 Mar 1;168(1):209-224. doi: 10.1093/toxsci/kfy294. Toxicol Sci. 2019. PMID: 30517741 Free PMC article.
-
Protoporphyrin IX: the Good, the Bad, and the Ugly.J Pharmacol Exp Ther. 2016 Feb;356(2):267-75. doi: 10.1124/jpet.115.228130. Epub 2015 Nov 20. J Pharmacol Exp Ther. 2016. PMID: 26588930 Free PMC article. Review.
-
N-Methyl Protoporphyrin IX: An Understudied Porphyrin.Chem Res Toxicol. 2022 Dec 19;35(12):2186-2193. doi: 10.1021/acs.chemrestox.2c00214. Epub 2022 Dec 2. Chem Res Toxicol. 2022. PMID: 36459538 Free PMC article. Review.
Cited by
-
Results of a pilot study of isoniazid in patients with erythropoietic protoporphyria.Mol Genet Metab. 2019 Nov;128(3):309-313. doi: 10.1016/j.ymgme.2019.07.017. Epub 2019 Jul 31. Mol Genet Metab. 2019. PMID: 31395332 Free PMC article.
-
Toxicoproteomic Profiling of hPXR Transgenic Mice Treated with Rifampicin and Isoniazid.Cells. 2020 Jul 9;9(7):1654. doi: 10.3390/cells9071654. Cells. 2020. PMID: 32660103 Free PMC article.
-
A Review of Fatty Acid Biosynthesis Enzyme Inhibitors as Promising Antimicrobial Drugs.Pharmaceuticals (Basel). 2023 Mar 10;16(3):425. doi: 10.3390/ph16030425. Pharmaceuticals (Basel). 2023. PMID: 36986522 Free PMC article. Review.
-
The Opportunities of Metabolomics in Drug Safety Evaluation.Curr Pharmacol Rep. 2017 Feb;3(1):10-15. doi: 10.1007/s40495-016-0079-5. Epub 2017 Jan 3. Curr Pharmacol Rep. 2017. PMID: 28758057 Free PMC article.
-
Deficiency of N-acetyltransferase increases the interactions of isoniazid with endobiotics in mouse liver.Biochem Pharmacol. 2017 Dec 1;145:218-225. doi: 10.1016/j.bcp.2017.09.001. Epub 2017 Sep 6. Biochem Pharmacol. 2017. PMID: 28888949 Free PMC article.
References
-
- World Health Organisation. Global tuberculosis report. 2015.
-
- Centers for Disease Control and Prevention. Severe isoniazid-associated liver injuries among persons being treated for latent tuberculosis infection - United States, 2004–2008. MMWR Morb Mortal Wkly Rep. 2010;59:224–229. - PubMed
-
- Mitchell JR, Zimmerman HJ, Ishak KG, Thorgeirsson UP, Timbrell JA, Snodgrass WR, Nelson SD. Isoniazid liver injury: clinical spectrum, pathology, and probable pathogenesis. Ann Intern Med. 1976;84:181–192. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

