Immunological Cross-Reactivity between Malaria Vaccine Target Antigen P48/45 in Plasmodium vivax and P. falciparum and Cross-Boosting of Immune Responses
- PMID: 27438603
- PMCID: PMC4954667
- DOI: 10.1371/journal.pone.0158212
Immunological Cross-Reactivity between Malaria Vaccine Target Antigen P48/45 in Plasmodium vivax and P. falciparum and Cross-Boosting of Immune Responses
Abstract
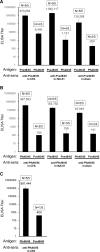
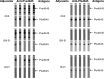
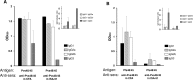
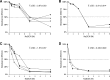
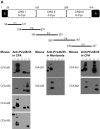
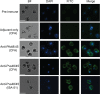
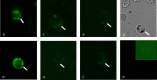
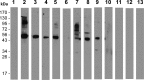
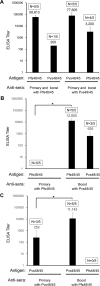
In general, malaria immunity has been suggested to be species specific with very little, if any, known cross-reactivity between Plasmodium vivax and P. falciparum, both of which are responsible for >90% of human malaria, and co-endemic in many countries. It is therefore believed that species-specific immunity may be needed to target different species of Plasmodium. Pfs48/45 and Pvs48/45 are well established targets in the sexual stages of the malaria parasites, and are being pursued for the development of transmission blocking vaccines. Comparison of their sequences reveals 61% and 55% identity at the DNA and protein level, respectively raising the possibility that these two target antigens might share cross-reacting epitopes. Having succeeded in expressing recombinant Pfs48/45 and Pvs48/45 proteins, we hypothesized that these proteins will not only exhibit immunological cross-reactivity but also cross-boost immune responses. Mice were immunized with purified recombinant proteins using CFA, Montanide ISA-51 and alum as adjuvants, and the sera were analyzed by ELISA, Western blotting and indirect fixed and live IFA to address the hypothesis. Our studies revealed that Pvs48/45-immune sera showed strong cross-reactivity to full length Pfs48/45 protein, and the majority of this cross reactivity was in the amino-terminal and carboxyl-terminal sub-fragments of Pfs48/45. In cross-boosting experiments Pfs48/45 and Pvs48/45 antigens were able to cross-boost each other in mouse immunization studies. Additionally we also noticed an effect of adjuvants in the overall magnitude of observed cross-reactivity. These studies may have significant implications for immunity targeting transmission of both the species of malaria parasites.
Conflict of interest statement
Figures
Similar articles
-
Antibodies elicited during natural infection in a predominantly Plasmodium falciparum transmission area cross-react with sexual stage-specific antigen in P. vivax.Acta Trop. 2017 Jun;170:105-111. doi: 10.1016/j.actatropica.2017.02.032. Epub 2017 Feb 28. Acta Trop. 2017. PMID: 28257812 Free PMC article.
-
Evaluation of combination vaccines targeting transmission of Plasmodium falciparum and P. vivax.Vaccine. 2024 Aug 30;42(21):126140. doi: 10.1016/j.vaccine.2024.07.041. Epub 2024 Jul 20. Vaccine. 2024. PMID: 39033079
-
Cross-reactivity of rPvs48/45, a recombinant Plasmodium vivax protein, with plasma from Plasmodium falciparum endemic areas of Africa.PLoS One. 2025 Mar 18;20(3):e0302605. doi: 10.1371/journal.pone.0302605. eCollection 2025. PLoS One. 2025. PMID: 40100850 Free PMC article.
-
Immunity against sexual stage Plasmodium falciparum and Plasmodium vivax parasites.Immunol Rev. 2020 Jan;293(1):190-215. doi: 10.1111/imr.12828. Epub 2019 Dec 16. Immunol Rev. 2020. PMID: 31840844 Free PMC article. Review.
-
Transmission-Blocking Vaccines: Harnessing Herd Immunity for Malaria Elimination.Expert Rev Vaccines. 2021 Feb;20(2):185-198. doi: 10.1080/14760584.2021.1878028. Epub 2021 Jan 31. Expert Rev Vaccines. 2021. PMID: 33478283 Free PMC article. Review.
Cited by
-
Structural analysis of Plasmodium falciparum ookinete surface antigen Pfs28 relevant for malaria vaccine design.Sci Rep. 2022 Nov 15;12(1):19556. doi: 10.1038/s41598-022-24054-3. Sci Rep. 2022. PMID: 36379968 Free PMC article.
-
Functional Conservation of P48/45 Proteins in the Transmission Stages of Plasmodium vivax (Human Malaria Parasite) and P. berghei (Murine Malaria Parasite).mBio. 2018 Sep 4;9(5):e01627-18. doi: 10.1128/mBio.01627-18. mBio. 2018. PMID: 30181253 Free PMC article.
-
Transmission-reducing and -enhancing monoclonal antibodies against Plasmodium vivax gamete surface protein Pvs48/45.Infect Immun. 2024 Mar 12;92(3):e0037423. doi: 10.1128/iai.00374-23. Epub 2024 Jan 30. Infect Immun. 2024. PMID: 38289124 Free PMC article.
-
Identification of linear epitopes on the flagellar proteins of Clostridioides difficile.Sci Rep. 2021 May 11;11(1):9940. doi: 10.1038/s41598-021-89488-7. Sci Rep. 2021. PMID: 33976336 Free PMC article.
-
Plasmodium 6-Cysteine Proteins: Functional Diversity, Transmission-Blocking Antibodies and Structural Scaffolds.Front Cell Infect Microbiol. 2022 Jul 8;12:945924. doi: 10.3389/fcimb.2022.945924. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35899047 Free PMC article. Review.
References
-
- Quakyi IA, Carter R, Rener J, Kumar N, Good MF, Miller LH. The 230-kDa gamete surface protein of Plasmodium falciparum is also a target for transmission-blocking antibodies. J Immunol. 1987;139(12):4213–7. - PubMed
-
- Arévalo-Herrera M, Vallejo AF, Rubiano K, Solarte Y, Marin C, Castellanos A, et al. Recombinant Pvs48/45 Antigen Expressed in E. coli Generates Antibodies that Block Malaria Transmission in Anopheles albimanus Mosquitoes. PloS one. 2015;10(3):e0119335 10.1371/journal.pone.0119335 - DOI - PMC - PubMed
-
- Kaslow DC, Quakyi IA, Syin C, Raum MG, Keister DB, Coligan JE, et al. A vaccine candidate from the sexual stage of human malaria that contains EGF-like domains. Nature. 1988;333(6168):74–6. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

