Chicken volatiles repel host-seeking malaria mosquitoes
- PMID: 27439360
- PMCID: PMC4955153
- DOI: 10.1186/s12936-016-1386-3
Chicken volatiles repel host-seeking malaria mosquitoes
Abstract
Background: Anopheles arabiensis is a dominant vector of malaria in sub-Saharan Africa, which feeds indoors and outdoors on human and other vertebrate hosts, making it a difficult species to control with existing control methods. Novel methods that reduce human-vector interactions are, therefore, required to improve the impact of vector control programmes. Investigating the mechanisms underlying the host discrimination process in An. arabiensis could provide valuable knowledge leading to the development of novel control technologies. In this study, a host census and blood meal analysis were conducted to determine the host selection behaviour of An. arabiensis. Since mosquitoes select and discriminate among hosts primarily using olfaction, the volatile headspace of the preferred non-human host and non-host species, were collected. Using combined gas chromatography and electroantennographic detection analysis followed by combined gas chromatography and mass spectrometry, the bioactive compounds in the headspace collections were identified. The efficiency of the identified non-host compounds to repel host-seeking malaria mosquitoes was tested under field conditions.
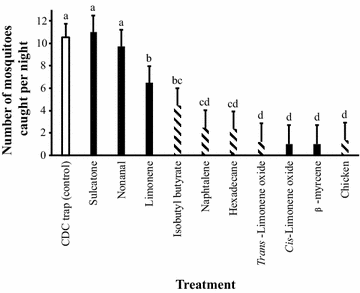
Results: The host census and blood meal analyses demonstrated that An. arabiensis strongly prefers human blood when host seeking indoors, while it randomly feeds on cattle, goats and sheep when found outdoors. However, An. arabiensis avoids chickens despite their relatively high abundance, indicating that chickens are a non-host species for this vector. Eleven bioactive compounds were found in the headspace of the non-host species. Six of these were species-specific, out of which four were identified using combined gas chromatography and mass spectrometry. When tested in the field, the chicken-specific compounds, isobutyl butyrate, naphthalene, hexadecane and trans-limonene oxide, and the generic host compounds, limonene, cis-limonene oxide and β-myrcene, significantly reduced trap catches within the house compared to a negative control. A significant reduction in trap catch was also observed when suspending a caged chicken next to the trap.
Conclusions: Non-host volatiles repel host-seeking An. arabiensis and thus play a significant role in host discrimination. As such, this study demonstrates that non-host volatiles can provide protection to humans at risk of mosquito-vectored diseases in combination with established control programmes.
Keywords: Anopheles arabiensis; Blood meal analysis; Host discrimination; Host species abundance; Non-host volatiles.
Figures

Similar articles
-
Smelly interactions: host-borne volatile organic compounds triggering behavioural responses in mosquitoes, sand flies, and ticks.Parasit Vectors. 2024 May 16;17(1):227. doi: 10.1186/s13071-024-06299-1. Parasit Vectors. 2024. PMID: 38755646 Free PMC article. Review.
-
A crossover study to evaluate the diversion of malaria vectors in a community with incomplete coverage of spatial repellents in the Kilombero Valley, Tanzania.Parasit Vectors. 2016 Aug 15;9:451. doi: 10.1186/s13071-016-1738-4. Parasit Vectors. 2016. PMID: 27527601 Free PMC article.
-
Blood-feeding behaviour of the malarial mosquito Anopheles arabiensis: implications for vector control.Med Vet Entomol. 2006 Dec;20(4):425-37. doi: 10.1111/j.1365-2915.2006.652.x. Med Vet Entomol. 2006. PMID: 17199754
-
Feeding and resting behaviour of malaria vector, Anopheles arabiensis with reference to zooprophylaxis.Malar J. 2007 Jul 30;6:100. doi: 10.1186/1475-2875-6-100. Malar J. 2007. PMID: 17663787 Free PMC article.
-
Chemical signaling in mosquito-host interactions: the role of human skin microbiota.Curr Opin Insect Sci. 2017 Apr;20:68-74. doi: 10.1016/j.cois.2017.03.011. Epub 2017 Apr 10. Curr Opin Insect Sci. 2017. PMID: 28602238 Review.
Cited by
-
Host Preferences and Impact of Climate on Blood Feeding in Anopheles funestus Group from South Africa.Trop Med Infect Dis. 2024 Oct 21;9(10):251. doi: 10.3390/tropicalmed9100251. Trop Med Infect Dis. 2024. PMID: 39453278 Free PMC article.
-
Smelly interactions: host-borne volatile organic compounds triggering behavioural responses in mosquitoes, sand flies, and ticks.Parasit Vectors. 2024 May 16;17(1):227. doi: 10.1186/s13071-024-06299-1. Parasit Vectors. 2024. PMID: 38755646 Free PMC article. Review.
-
Modulation of odour-guided behaviour in mosquitoes.Cell Tissue Res. 2021 Jan;383(1):195-206. doi: 10.1007/s00441-020-03368-6. Epub 2021 Jan 23. Cell Tissue Res. 2021. PMID: 33486608 Free PMC article. Review.
-
Evaluation of Host-Derived Volatiles for Trapping Culicoides Biting Midges (Diptera: Ceratopogonidae).J Chem Ecol. 2017 Jul;43(7):662-669. doi: 10.1007/s10886-017-0860-x. Epub 2017 Jul 3. J Chem Ecol. 2017. PMID: 28674827 Free PMC article.
-
Plasmodium-associated changes in human odor attract mosquitoes.Proc Natl Acad Sci U S A. 2018 May 1;115(18):E4209-E4218. doi: 10.1073/pnas.1721610115. Epub 2018 Apr 16. Proc Natl Acad Sci U S A. 2018. PMID: 29666273 Free PMC article. Clinical Trial.
References
-
- WHO . World malaria report. Geneva: World Health Organization; 2014.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

