Intravenous immunoglobulin therapy for small fiber neuropathy: study protocol for a randomized controlled trial
- PMID: 27439408
- PMCID: PMC4955261
- DOI: 10.1186/s13063-016-1450-x
Intravenous immunoglobulin therapy for small fiber neuropathy: study protocol for a randomized controlled trial
Abstract
Background: Small fiber neuropathy is the most common cause of neuropathic pain in peripheral neuropathies, with a minimum prevalence of 53/100,000. Patients experience excruciating pain, and currently available anti-neuropathic and other pain drugs do not relieve the pain substantially. Several open-label studies have suggested an immunological basis in small fiber neuropathy and have reported efficacy of treatment with intravenous immunoglobulin. Therefore, immunological mechanisms conceivably may play a role in small fiber neuropathy. To date, no randomized controlled study with intravenous immunoglobulin in patients with small fiber neuropathy has been performed.
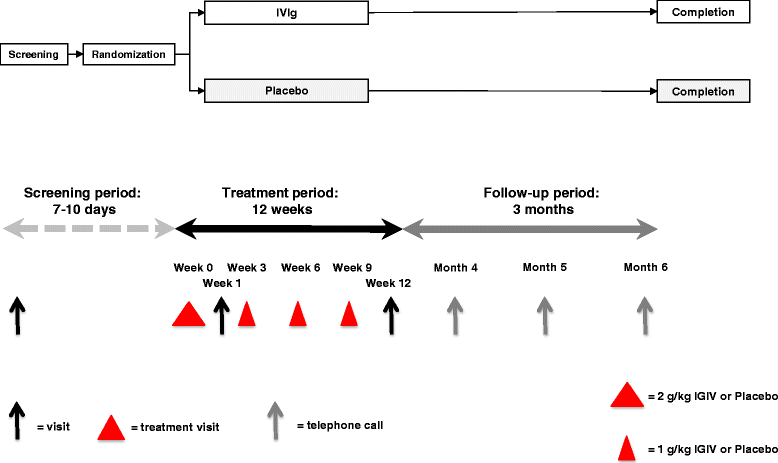
Methods/design: This study is a randomized, double-blind, placebo-controlled, clinical trial in patients with idiopathic small fiber neuropathy. The primary objective is to investigate the efficacy of intravenous immunoglobulin versus placebo on pain alleviation. A 1-point change in the PI-NRS compared to baseline is considered the minimum clinically important difference. In the IVIg-treated group, we assume a response rate of approximately 60 % based on the criteria composed by the IMMPACT group for measurement of pain. Based on this, a sample size of 60 patients is needed. Eligible patients fulfilling the inclusion/exclusion criteria will be randomized to receive either intravenous immunoglobulin or placebo (0.9 % saline). The treatment regimen will start with a loading dose of 2 g/kg body weight over 2-4 consecutive days, followed by a maintenance dose of 1 g/kg body weight over 1-2 consecutive days given three times at a 3-week interval. The primary endpoint is the comparison of the percentage of responder subjects between the two treatment groups from the first randomization during the 12 weeks of treatment. A responder is defined as ≥ 1-point Pain Intensity Numerical Rating Scale improvement on the mean weekly peak pain relative to baseline. The secondary outcomes are pain intensity, pain qualities, other small fiber neuropathy-related complaints, daily and social functioning, as well as quality of life. In addition, safety assessments will be performed for adverse events, vital signs, and laboratory values outside the normal range. Responders during the 12-week treatment period will be followed during a 3-month extension phase.
Discussion: This is the first randomized, double-blind, placebo-controlled clinical trial with intravenous immunoglobulin in patients with idiopathic small fiber neuropathy. Positive findings will result in a new treatment option for small fiber neuropathy and support an immunological role in this condition.
Trial registration: ClinicalTrials.gov, NCT02637700 . Registered on 16 December 2015.
Keywords: Immunology; Intravenous immunoglobulin; Painful neuropathy; Randomized controlled trial; Small fiber neuropathy.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous