Selective laser melting of titanium alloy enables osseointegration of porous multi-rooted implants in a rabbit model
- PMID: 27439427
- PMCID: PMC4955147
- DOI: 10.1186/s12938-016-0207-9
Selective laser melting of titanium alloy enables osseointegration of porous multi-rooted implants in a rabbit model
Abstract
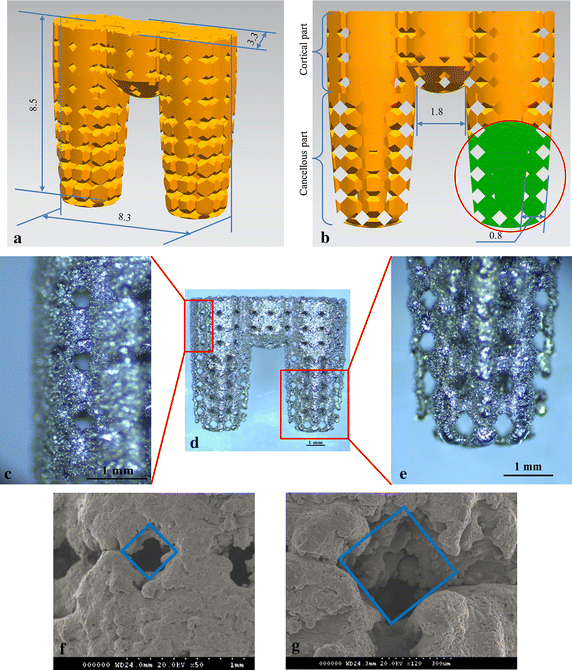
Background: Osseointegration refers to the direct connection between living bone and the surface of a load-bearing artificial implant. Porous implants with well-controlled porosity and pore size can enhance osseointegration. However, until recently implants were produced by machining solid core titanium rods. The aim of this study was to develop a multi-rooted dental implant (MRI) with a connected porous surface structure to facilitate osseointegration.
Methods: MRIs manufactured by selective laser melting (SLM) and commercial implants with resorbable blasting media (RBM)-treated surfaces were inserted into the hind limbs of New Zealand white rabbits. Osseointegration was evaluated periodically over 12 weeks by micro-computerized tomography (CT) scanning, histological analysis, mechanical push-out tests, and torque tests.
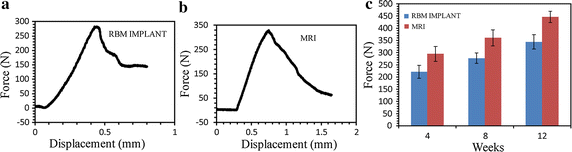
Results: Bone volume densities were consistently higher in the MRI group than in the RBM group throughout the study period, ultimately resulting in a peak value of 48.41 % for the MRI group. Histological analysis revealed denser surrounding bone growth in the MRIs; after 4 and 8 weeks, bone tissue had grown into the pore structures and root bifurcation areas, respectively. Biomechanics tests indicated binding of the porous MRIs to the neobone tissues, as push-out forces strengthened from 294.7 to 446.5 N and maximum mean torque forces improved from 81.15 to 289.57 N (MRI), versus 34.79 to 87.8 N in the RBM group.
Conclusions: MRIs manufactured by SLM possess a connected porous surface structure that improves the osteogenic characteristics of the implant surface.
Keywords: Biomechanics; Implant design; Multi-rooted implant; Osseointegration; Titanium (alloys).
Figures





References
-
- Chappuis V, Buser R, Brägger U, Bornstein MM, Salvi GE, Buser D. Long-term outcomes of dental implants with a titanium plasma-sprayed surface: a 20-year prospective case series study in partially edentulous patients. Clin Implant Dent Relat Res. 2013;15:780–790. doi: 10.1111/cid.12056. - DOI - PubMed
-
- Fugazzotto PA, Vlassis J. Long-term success of sinus augmentation using various surgical approaches and grafting materials. Int J Oral Maxillofac Implant. 1998;13:52–58. - PubMed
-
- Sisti A, Canullo L, Mottola MP, Covani U, Barone A, Botticelli D. Clinical evaluation of a ridge augmentation procedure for the severely resorbed alveolar socket: multicenter randomized controlled trial, preliminary results. Clin Oral Implant Res. 2012;23:526–535. doi: 10.1111/j.1600-0501.2011.02386.x. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

