Nevirapine- Versus Lopinavir/Ritonavir-Based Antiretroviral Therapy in HIV-Infected Infants and Young Children: Long-term Follow-up of the IMPAACT P1060 Randomized Trial
- PMID: 27439527
- PMCID: PMC5036919
- DOI: 10.1093/cid/ciw488
Nevirapine- Versus Lopinavir/Ritonavir-Based Antiretroviral Therapy in HIV-Infected Infants and Young Children: Long-term Follow-up of the IMPAACT P1060 Randomized Trial
Abstract
Background: The International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT) P1060 study demonstrated short-term superiority of lopinavir/ritonavir (LPV/r) over nevirapine (NVP) in antiretroviral therapy (ART), regardless of prior NVP exposure. However, NVP-based ART had a marginal benefit in CD4 percentage (CD4%) and growth. We compared 5-year outcomes from this clinical trial.
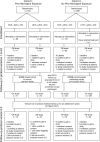
Methods: Human immunodeficiency virus (HIV)-infected, ART-eligible children were enrolled into 2 cohorts based on prior NVP exposure and randomized to NVP- or LPV/r-based ART. The data safety monitoring board recommended unblinding results in both cohorts due to superiority of LPV/r for the primary endpoint: stopping randomized treatment, virologic failure (VF), or death by 6 months. Participants were offered a switch in regimens (if on NVP) and continued observational follow-up. We compared time to VF or death, death, and CD4% and growth changes using intention-to-treat analyses. Additionally, inverse probability weights were used to account for treatment switching and censoring.
Results: As of September 2014, 329 of the 451 (73%) enrolled participants were still in follow-up (median, 5.3 years; interquartile range [IQR], 4.3-6.4), with 52% on NVP and 88% on LPV/r as originally randomized. NVP arm participants had significantly higher risk of VF or death (adjusted hazard ratio [aHR], 1.90; 95% confidence interval [CI], 1.37-2.65) but not death alone (aHR, 1.65; 95% CI, .72-3.76) compared with participants randomized to LPV/r. Mean CD4% was significantly higher in the NVP arm up to 1 year after ART initiation, but not beyond. Mean weight-for-age z scores were marginally higher in the NVP arm, but height-for-age z scores did not differ. Similar trends were observed in sensitivity analyses.
Conclusions: These findings support the current World Health Organization recommendation of LPV/r in first-line ART regimens for HIV-infected children.
Clinical trials registration: NCT00307151.
Keywords: HIV/AIDS; antiretroviral therapy; long-term follow-up; pediatrics.
© The Author 2016. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail journals.permissions@oup.com.
Figures


References
-
- Newell ML, Coovadia H, Cortina-Borja M, Rollins N, Gaillard P, Dabis F. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet 2004; 364:1236–43. - PubMed
-
- World Health Organization. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV, September 2015. Available at: http://apps.who.int/iris/bitstream/10665/186275/1/9789241509565_eng.pdf Accessed 31 July 2016. - PubMed
-
- Eshleman SH, Mracna M, Guay LA et al. . Selection and fading of resistance mutations in women and infants receiving nevirapine to prevent HIV-1 vertical transmission (HIVNET 012). AIDS 2001; 15:1951–7. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

