Accuracy, reliability, feasibility and nurse acceptance of a subcutaneous continuous glucose management system in critically ill patients: a prospective clinical trial
- PMID: 27439710
- PMCID: PMC4954792
- DOI: 10.1186/s13613-016-0167-z
Accuracy, reliability, feasibility and nurse acceptance of a subcutaneous continuous glucose management system in critically ill patients: a prospective clinical trial
Abstract
Background: Continuous glucose monitoring (CGM) has not yet been implemented in the intensive care unit (ICU) setting. The purpose of this study was to evaluate reliability, feasibility, nurse acceptance and accuracy of the Medtronic Sentrino(®) CGM system in critically ill patients.
Methods: Sensors were inserted into the subcutaneous tissue of the patient's thigh, quantifying interstitial glucose concentration for up to 72 h per sensor. Reliability and feasibility analysis included frequency of data display, data gaps and reasons for sensor removal. We surveyed nurse acceptance in a questionnaire. For the accuracy analysis, we compared sensor values to glucose values obtained via blood gas analysis. Potential benefits of CGM were investigated in intra-individual analyses of factors, such as glycemic variability or time in target range achieved with CGM compared to that achieved with intermittent glucose monitoring.
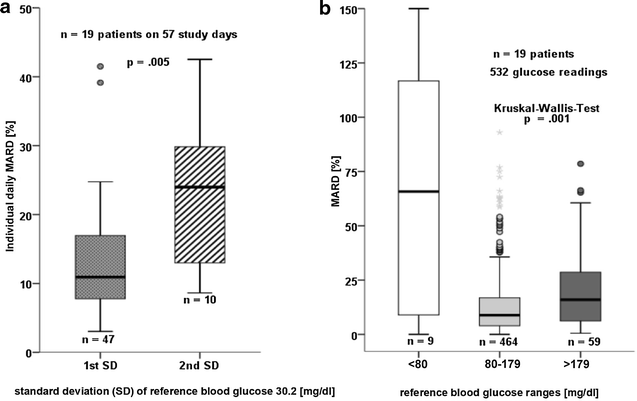
Results: The device generated 68,655 real-time values from 31 sensors in 20 critically ill patients. 532 comparative blood glucose values were collected. Data were displayed during 32.5 h [16.0/62.4] per sensor, which is 45.1 % of the expected time of 72 h and 84.8 % of 37.9 h actual monitoring time. 21 out of 31 sensors were removed prematurely. 79.1 % of the nursing staff rated the device as not beneficial; the response rate was one-third. Mean absolute relative difference was 15.3 % (CI 13.5-17.0 %). Clarke error grid: 76.9 % zone A, 21.6 % zone B, 0.2 % zone C, 0.9 % zone D, 0.4 % zone E. Bland-Altman plot: mean bias +0.53 mg/dl, limits of agreement +64.6 and -63.5 mg/dl. Accuracy deteriorated during elevated glycemic variability and in the hyperglycemic range. There was no reduction in dysglycemic events during CGM compared to 72 h before and after CGM. If CGM was measuring accurately, it identified more hyperglycemic events when compared to intermittent measurements. This study was not designed to evaluate potential benefits of CGM on glucose control.
Conclusions: The subcutaneous CGM system did not perform with satisfactory accuracy, feasibility, or nursing acceptance when evaluated in 20 medical-surgical ICU patients. Low point accuracy and prolonged data gaps significantly limited the potential clinical usefulness of the CGM trend data. Accurate continuous data display, with a MARD < 14 %, showed potential benefits in a subgroup of our patients. Trial registration NCT02296372; Ethic vote Charité EA2/095/14.
Keywords: Accuracy; Continuous glucose monitoring; Critically ill patients; Evaluation; Feasibility; ICU; Interstitial; Medtronic Sentrino®; Nurse acceptance; Reliability; Subcutaneous.
Figures



References
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

