Dynamics of gamete production and mating in the parasitic protist Trypanosoma brucei
- PMID: 27439767
- PMCID: PMC4955137
- DOI: 10.1186/s13071-016-1689-9
Dynamics of gamete production and mating in the parasitic protist Trypanosoma brucei
Abstract
Background: Sexual reproduction in Plasmodium falciparum and Trypanosoma brucei occurs in the insect vector and is important in generating hybrid strains with different combinations of parental characteristics. Production of hybrid parasite genotypes depends on the likelihood of co-infection of the vector with multiple strains. In mosquitoes, existing infection with Plasmodium facilitates the establishment of a second infection, although the asynchronicity of gamete production subsequently prevents mating. In the trypanosome/tsetse system, flies become increasingly refractory to infection as they age, so the likelihood of a fly acquiring a second infection also decreases. This effectively restricts opportunities for trypanosome mating to co-infections picked up by the fly on its first feed, unless an existing infection increases the chance of successful second infection as in the Plasmodium/mosquito system.
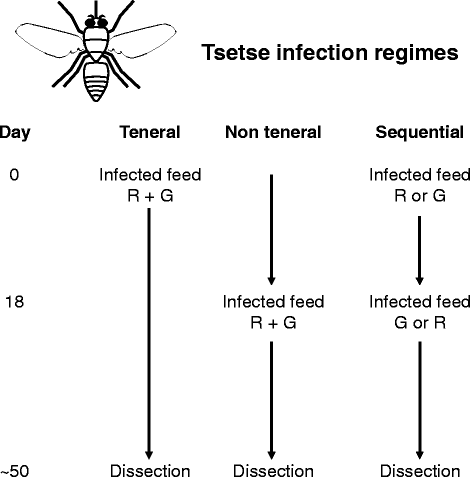
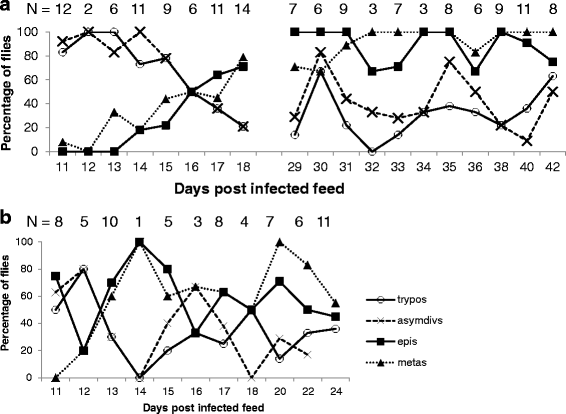
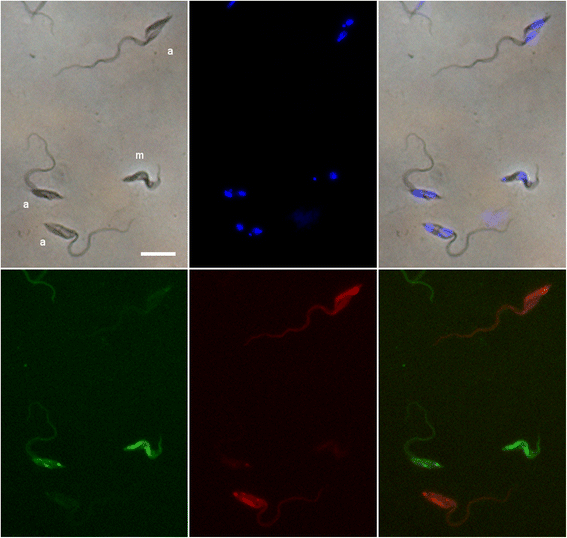
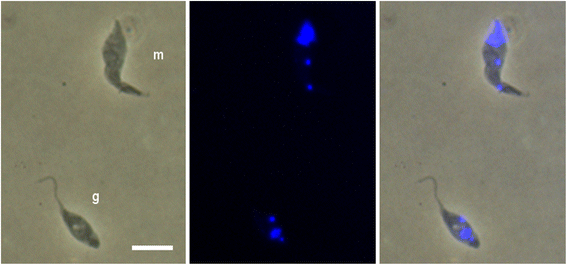
Results: Using green and red fluorescent trypanosomes, we compared the rates of trypanosome infection and hybrid production in flies co-infected on the first feed, co-infected on a subsequent feed 18 days after emergence, or fed sequentially with each trypanosome clone 18 days apart. Infection rates were highest in the midguts and salivary glands (SG) of flies that received both trypanosome clones in their first feed, and were halved when the infected feed was delayed to day 18. In flies fed the two trypanosome clones sequentially, the second clone often failed to establish a midgut infection and consequently was not present in the SG. Nevertheless, hybrids were recovered from all three groups of infected flies. Meiotic stages and gametes were produced continuously from day 11 to 42 after the infective feed, and in sequentially infected flies, the co-occurrence of gametes led to hybrid formation.
Conclusions: We found that a second trypanosome strain can establish infection in the tsetse SG 18 days after the first infected feed, with co-mingling of gametes and production of trypanosome hybrids. Establishment of the second strain was severely compromised by the strong immune response of the fly to the existing infection. Although sequential infection provides an opportunity for trypanosome mating, the easiest way for a tsetse fly to acquire a mixed infection is by feeding on a co-infected host.
Keywords: Competition; Human infectivity; Hybrids; Immune response; Mating; Sequential infection; Sexual reproduction; Trypanosoma; Tsetse.
Figures

References
-
- Buxton PA. The natural history of tsetse flies. Memoir 10 London School of Hygiene and Tropical Medicine. London: HK Lewis; 1955.
-
- Welburn SC, Maudlin I. The nature of the teneral state in Glossina and its role in the acquisition of trypanosome infection in tsetse. Ann Trop Med Parasit. 1992;86(5):529–536. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

