Constituents and functional implications of the rat default mode network
- PMID: 27439860
- PMCID: PMC4978262
- DOI: 10.1073/pnas.1601485113
Constituents and functional implications of the rat default mode network
Abstract
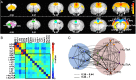
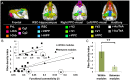
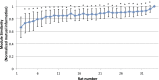
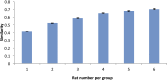
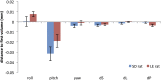
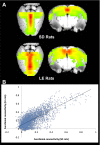
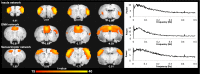
The default mode network (DMN) has been suggested to support a variety of self-referential functions in humans and has been fractionated into subsystems based on distinct responses to cognitive tasks and functional connectivity architecture. Such subsystems are thought to reflect functional hierarchy and segregation within the network. Because preclinical models can inform translational studies of neuropsychiatric disorders, partitioning of the DMN in nonhuman species, which has previously not been reported, may inform both physiology and pathophysiology of the human DMN. In this study, we sought to identify constituents of the rat DMN using resting-state functional MRI (rs-fMRI) and diffusion tensor imaging. After identifying DMN using a group-level independent-component analysis on the rs-fMRI data, modularity analyses fractionated the DMN into an anterior and a posterior subsystem, which were further segregated into five modules. Diffusion tensor imaging tractography demonstrates a close relationship between fiber density and the functional connectivity between DMN regions, and provides anatomical evidence to support the detected DMN subsystems. Finally, distinct modulation was seen within and between these DMN subcomponents using a neurocognitive aging model. Taken together, these results suggest that, like the human DMN, the rat DMN can be partitioned into several subcomponents that may support distinct functions. These data encourage further investigation into the neurobiological mechanisms of DMN processing in preclinical models of both normal and disease states.
Keywords: aging; default mode network; functional connectivity; modularity; rat brain.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Vincent JL, et al. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447(7140):83–86. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

