Effective adoptive immunotherapy of triple-negative breast cancer by folate receptor-alpha redirected CAR T cells is influenced by surface antigen expression level
- PMID: 27439908
- PMCID: PMC4955216
- DOI: 10.1186/s13045-016-0285-y
Effective adoptive immunotherapy of triple-negative breast cancer by folate receptor-alpha redirected CAR T cells is influenced by surface antigen expression level
Abstract
Background: The poor prognosis and the limited efficacy of targeted therapy in patients with triple-negative breast cancer (TNBC) have raised the need for alternative therapies. Recent studies have demonstrated that folate receptor-alpha (FRα) may represent an ideal tumor-associated marker for immunotherapy for TNBC.
Methods: The aim of the present study was to apply a chimeric antigen receptor (CAR) approach for the targeting of FRα expressed on TNBC cells and evaluate the antitumor activity of CAR-engineered T cells in vitro and in vivo.
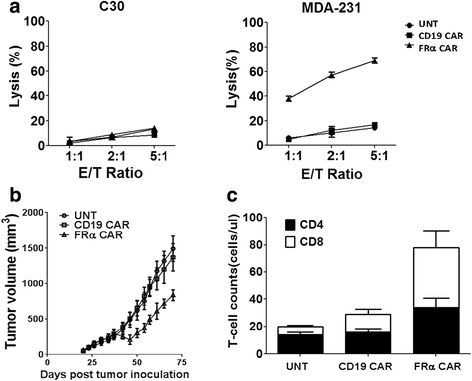
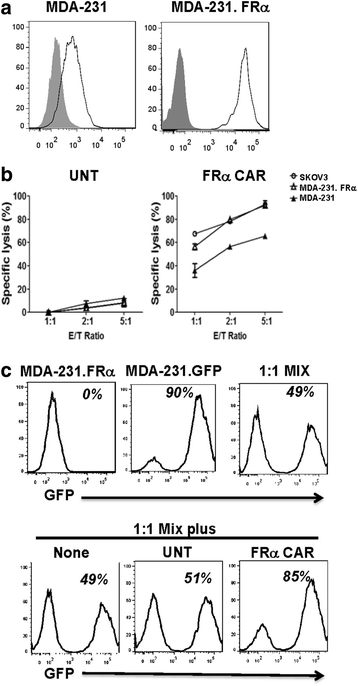
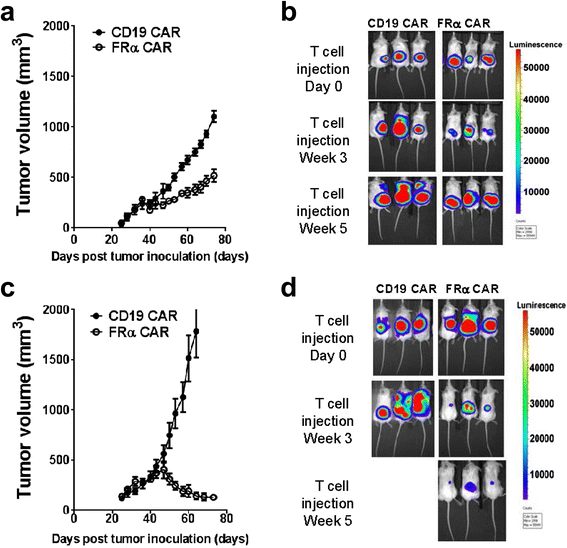
Results: We found that human T cells expressing a FRα-specific CAR were potent and specific killers of TNBC cells that express moderate levels of FRα in vitro and significantly inhibited tumor outgrowth following infusion into immunodeficient mice bearing an MDA-MB-231 tumor xenograft. However, the antitumor activity of the FRα CAR T cells was modest when compared to the same CAR T cells applied in an ovarian tumor xenograft model where FRα expression is more abundant. Notably, FRα CAR T cells induced superior tumor regression in vivo against MDA-MB-231 that was engineered for overexpression of FRα.
Conclusions: Taken together, our results show that FRα CAR T cells can mediate antitumor activity against established TNBC tumor, particularly when FRα is expressed at higher levels. These results have significant implications for the pre-selection of patients with high antigen expression levels when utilizing CAR-based adoptive T cell therapies of cancer in future clinical trials.
Keywords: Chimeric antigen receptor; Folate receptor-alpha; Immunotherapy; Triple-negative breast cancer.
Figures
Similar articles
-
In vivo persistence, tumor localization, and antitumor activity of CAR-engineered T cells is enhanced by costimulatory signaling through CD137 (4-1BB).Cancer Res. 2011 Jul 1;71(13):4617-27. doi: 10.1158/0008-5472.CAN-11-0422. Epub 2011 May 5. Cancer Res. 2011. PMID: 21546571 Free PMC article.
-
Modification of cytokine-induced killer cells with folate receptor alpha (FRα)-specific chimeric antigen receptors enhances their antitumor immunity toward FRα-positive ovarian cancers.Mol Immunol. 2017 May;85:293-304. doi: 10.1016/j.molimm.2017.03.017. Epub 2017 Mar 27. Mol Immunol. 2017. PMID: 28360017
-
Fourth-generation chimeric antigen receptor T cells targeting folate receptor alpha antigen expressed on breast cancer cells for adoptive T cell therapy.Breast Cancer Res Treat. 2021 Feb;186(1):25-36. doi: 10.1007/s10549-020-06032-3. Epub 2021 Jan 3. Breast Cancer Res Treat. 2021. PMID: 33389403
-
CAR T-cell therapy for triple-negative breast cancer: Where we are.Cancer Lett. 2020 Oct 28;491:121-131. doi: 10.1016/j.canlet.2020.07.044. Epub 2020 Aug 12. Cancer Lett. 2020. PMID: 32795486 Review.
-
Emerging CAR-T Cell Therapy for the Treatment of Triple-Negative Breast Cancer.Mol Cancer Ther. 2020 Dec;19(12):2409-2421. doi: 10.1158/1535-7163.MCT-20-0385. Epub 2020 Oct 21. Mol Cancer Ther. 2020. PMID: 33087511 Review.
Cited by
-
Immunotherapy for Breast Cancer Treatment.Iran Biomed J. 2021 Mar 8;25(3):140-56. doi: 10.29252/ibj.25.3.140. Online ahead of print. Iran Biomed J. 2021. PMID: 33724757 Free PMC article.
-
EGFR-targeted CAR-T cells are potent and specific in suppressing triple-negative breast cancer both in vitro and in vivo.Clin Transl Immunology. 2020 May 3;9(5):e01135. doi: 10.1002/cti2.1135. eCollection 2020 May. Clin Transl Immunology. 2020. PMID: 32373345 Free PMC article.
-
Folate receptor alpha for cancer therapy: an antibody and antibody-drug conjugate target coming of age.MAbs. 2025 Dec;17(1):2470309. doi: 10.1080/19420862.2025.2470309. Epub 2025 Mar 5. MAbs. 2025. PMID: 40045156 Free PMC article. Review.
-
Immunotherapeutic interventions of Triple Negative Breast Cancer.J Transl Med. 2018 May 30;16(1):147. doi: 10.1186/s12967-018-1514-7. J Transl Med. 2018. PMID: 29848327 Free PMC article. Review.
-
Clinical trials of CAR-T cells in China.J Hematol Oncol. 2017 Oct 23;10(1):166. doi: 10.1186/s13045-017-0535-7. J Hematol Oncol. 2017. PMID: 29058636 Free PMC article.
References
-
- Rochman H, Selhub J, Karrison T. Folate binding protein and the estrogen receptor in breast cancer. Cancer Detect Prev. 1985;8(1-2):71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

