Assessment of the in vitro dermal irritation potential of cerium, silver, and titanium nanoparticles in a human skin equivalent model
- PMID: 27439971
- PMCID: PMC6191042
- DOI: 10.1080/15569527.2016.1211671
Assessment of the in vitro dermal irritation potential of cerium, silver, and titanium nanoparticles in a human skin equivalent model
Abstract
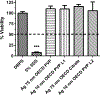
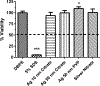
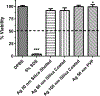
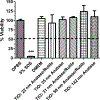
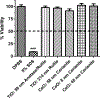
Metal nanoparticles can potentially contact human skin during their manufacture and use in commercial products. This study examined the potential of metal nanoparticles to elicit irritant contact dermatitis in a human skin equivalent model (HSEM) derived from keratinocytes. Ag (10-100 nm), TiO2 (22-214 nm), and CeO2 (15-40 nm) nanoparticles were studied. The Ag particles were either coated/shelled with silica or capped with citrate or polyvinylpyrrolidone and were in water. The TiO2 and CeO2 particles were suspended in media containing 10% fetal bovine serum. The particles (1 mg/ml) were applied to the epidermal surface of the HSEM. Positive (5% sodium dodecyl sulfate (SDS)) and negative controls (saline or media) were included. After 1-h exposure at 37 °C, the HSEM was washed with saline to remove the nanoparticles. Following a 42-h incubation (37 °C), HSEM viability was assessed using the MTT assay. A test substance is considered a dermal irritant if the HSEM viability is < 50%. The mean viability for the SDS-treated HSEM was 7.8%. The viabilities of the nanoparticle-treated HSEM were 91% or greater. The Ag, TiO2, and CeO2 nanoparticles examined were not dermal irritants under the conditions used in this study. The stratum corneum of the HSEM may limit penetration of metal nanoparticles to induce toxicity.
Keywords: Inorganic; irritation; metals; nanoparticles; skin.
Figures
Similar articles
-
Analysis for the potential of polystyrene and TiO2 nanoparticles to induce skin irritation, phototoxicity, and sensitization.Toxicol In Vitro. 2011 Dec;25(8):1863-9. doi: 10.1016/j.tiv.2011.05.022. Epub 2011 May 31. Toxicol In Vitro. 2011. PMID: 21664450
-
Evaluation of silver nanoparticle toxicity in skin in vivo and keratinocytes in vitro.Environ Health Perspect. 2010 Mar;118(3):407-13. doi: 10.1289/ehp.0901398. Epub 2009 Oct 23. Environ Health Perspect. 2010. PMID: 20064793 Free PMC article.
-
Skin Toxicity Assessment of Silver Nanoparticles in a 3D Epidermal Model Compared to 2D Keratinocytes.Int J Nanomedicine. 2019 Dec 9;14:9707-9719. doi: 10.2147/IJN.S225451. eCollection 2019. Int J Nanomedicine. 2019. PMID: 31849463 Free PMC article.
-
In vitro skin irritation: facts and future. State of the art review of mechanisms and models.Toxicol In Vitro. 2004 Jun;18(3):231-43. doi: 10.1016/j.tiv.2003.09.009. Toxicol In Vitro. 2004. PMID: 15046769 Review.
-
Irritants in combination with a synergistic or additive effect on the skin response: an overview of tandem irritation studies.Contact Dermatitis. 2006 Jun;54(6):303-12. doi: 10.1111/j.0105-1873.2006.00792.x. Contact Dermatitis. 2006. PMID: 16787451 Review.
Cited by
-
Antagonistic Skin Toxicity of Co-Exposure to Physical Sunscreen Ingredients Zinc Oxide and Titanium Dioxide Nanoparticles.Nanomaterials (Basel). 2022 Aug 12;12(16):2769. doi: 10.3390/nano12162769. Nanomaterials (Basel). 2022. PMID: 36014634 Free PMC article.
-
Toxicity Evaluation of TiO2 Nanoparticles on the 3D Skin Model: A Systematic Review.Front Bioeng Biotechnol. 2020 Jun 10;8:575. doi: 10.3389/fbioe.2020.00575. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32587852 Free PMC article.
-
In Vitro Dermal Safety Assessment of Silver Nanowires after Acute Exposure: Tissue vs. Cell Models.Nanomaterials (Basel). 2018 Apr 11;8(4):232. doi: 10.3390/nano8040232. Nanomaterials (Basel). 2018. PMID: 29641466 Free PMC article.
-
Influence of Titanium Dioxide Nanoparticles on Human Health and the Environment.Nanomaterials (Basel). 2021 Sep 10;11(9):2354. doi: 10.3390/nano11092354. Nanomaterials (Basel). 2021. PMID: 34578667 Free PMC article. Review.
-
Application of transgenic zebrafish for investigating inflammatory responses to nanomaterials: Recommendations for new users.F1000Res. 2025 Jun 23;12:51. doi: 10.12688/f1000research.128851.2. eCollection 2023. F1000Res. 2025. PMID: 40656220 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
