SPIRIT 2013 Statement: defining standard protocol items for clinical trials
- PMID: 27440100
- PMCID: PMC5114122
SPIRIT 2013 Statement: defining standard protocol items for clinical trials
Abstract
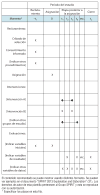
The protocol of a clinical trial serves as the foundation for study planning, conduct, reporting, and appraisal. However, trial protocols and existing protocol guidelines vary greatly in content and quality. This article describes the systematic development and scope of SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials) 2013, a guideline for the minimum content of a clinical trial protocol. The 33-item SPIRIT checklist applies to protocols for all clinical trials and focuses on content rather than format. The checklist recommends a full description of what is planned; it does not prescribe how to design or conduct a trial. By providing guidance for key content, the SPIRIT recommendations aim to facilitate the drafting of high-quality protocols. Adherence to SPIRIT would also enhance the transparency and completeness of trial protocols for the benefit of investigators, trial participants, patients, sponsors, funders, research ethics committees or institutional review boards, peer reviewers, journals, trial registries, policymakers, regulators, and other key stakeholders.
Conflict of interest statement
Conflictos de interés posibles. las declaraciones pueden verse en www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M12-1905
Figures
References
-
- Rennie D. Trial registration: a great idea switches from ignored to irresistible. JAMA. 2004;292:1359–62. - PubMed
-
- Strengthening the credibility of clinical research [artículo editorial] Lancet. 2010;375:1225. - PubMed
-
- Summerskill W, Collingridge D, Frankish H. Protocols, probity, and publication. Lancet. 2009;373:992. - PubMed
-
- Groves T. Let SPIRIT take you towards much clearer trial protocols. [Acceso el 1 de octubre de 2012];BMJ Group Blogs. 2009 Septiembre; Disponible en http://blogs.bmj.com/bmj/2009/09/25/trish-groves-let-spirittake-you-towa...
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical