Distinct effects of inflammation on preconditioning and regeneration of the adult zebrafish heart
- PMID: 27440424
- PMCID: PMC4967830
- DOI: 10.1098/rsob.160102
Distinct effects of inflammation on preconditioning and regeneration of the adult zebrafish heart
Abstract
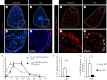
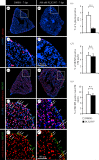
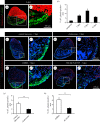
The adult heart is able to activate cardioprotective programmes and modifies its architecture in response to physiological or pathological changes. While mammalian cardiac remodelling often involves hypertrophic expansion, the adult zebrafish heart exploits hyperplastic growth. This capacity depends on the responsiveness of zebrafish cardiomyocytes to mitogenic signals throughout their entire life. Here, we have examined the role of inflammation on the stimulation of cell cycle activity in the context of heart preconditioning and regeneration. We used thoracotomy as a cardiac preconditioning model and cryoinjury as a model of cardiac infarction in the adult zebrafish. First, we performed a spatio-temporal characterization of leucocytes and cycling cardiac cells after thoracotomy. This analysis revealed a concomitance between the infiltration of inflammatory cells and the stimulation of the mitotic activity. However, decreasing the immune response using clodronate liposome injection, PLX3397 treatment or anti-inflammatory drugs surprisingly had no effect on the re-entry of cardiac cells into the cell cycle. In contrast, reducing inflammation using the same strategies after cryoinjury strongly impaired cardiac cell mitotic activity and the regenerative process. Taken together, our results show that, while the immune response is not necessary to induce cell-cycle activity in intact preconditioned hearts, inflammation is required for the regeneration of injured hearts in zebrafish.
Keywords: cardiac muscle; cardiomyocyte; cryoinjury; leucocytes; non-mammalian animal model; thoracotomy.
© 2016 The Authors.
Figures





Similar articles
-
Preconditioning boosts regenerative programmes in the adult zebrafish heart.Open Biol. 2016 Jul;6(7):160101. doi: 10.1098/rsob.160101. Open Biol. 2016. PMID: 27440423 Free PMC article.
-
A dual epimorphic and compensatory mode of heart regeneration in zebrafish.Dev Biol. 2015 Mar 1;399(1):27-40. doi: 10.1016/j.ydbio.2014.12.002. Epub 2014 Dec 31. Dev Biol. 2015. PMID: 25557620
-
Excessive inflammation impairs heart regeneration in zebrafish breakdance mutant after cryoinjury.Fish Shellfish Immunol. 2019 Jun;89:117-126. doi: 10.1016/j.fsi.2019.03.058. Epub 2019 Mar 27. Fish Shellfish Immunol. 2019. PMID: 30928664
-
Zebrafish heart regeneration: Factors that stimulate cardiomyocyte proliferation.Semin Cell Dev Biol. 2020 Apr;100:3-10. doi: 10.1016/j.semcdb.2019.09.005. Epub 2019 Sep 25. Semin Cell Dev Biol. 2020. PMID: 31563389 Free PMC article. Review.
-
Building and re-building the heart by cardiomyocyte proliferation.Development. 2016 Mar 1;143(5):729-40. doi: 10.1242/dev.132910. Development. 2016. PMID: 26932668 Free PMC article. Review.
Cited by
-
Pro-regenerative Dialogue Between Macrophages and Mesenchymal Stem/Stromal Cells in Osteoarthritis.Front Cell Dev Biol. 2021 Sep 17;9:718938. doi: 10.3389/fcell.2021.718938. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34604219 Free PMC article. Review.
-
The careg element reveals a common regulation of regeneration in the zebrafish myocardium and fin.Nat Commun. 2017 May 3;8:15151. doi: 10.1038/ncomms15151. Nat Commun. 2017. PMID: 28466843 Free PMC article.
-
Reciprocal analyses in zebrafish and medaka reveal that harnessing the immune response promotes cardiac regeneration.Elife. 2017 Jun 20;6:e25605. doi: 10.7554/eLife.25605. Elife. 2017. PMID: 28632131 Free PMC article.
-
Reactive oxygen species during heart regeneration in zebrafish: Lessons for future clinical therapies.Wound Repair Regen. 2021 Mar;29(2):211-224. doi: 10.1111/wrr.12892. Epub 2021 Jan 20. Wound Repair Regen. 2021. PMID: 33471940 Free PMC article. Review.
-
In Vivo Characterization of Endogenous Cardiovascular Extracellular Vesicles in Larval and Adult Zebrafish.Arterioscler Thromb Vasc Biol. 2021 Sep;41(9):2454-2468. doi: 10.1161/ATVBAHA.121.316539. Epub 2021 Jul 15. Arterioscler Thromb Vasc Biol. 2021. PMID: 34261327 Free PMC article.
References
-
- de Preux Charles A-S, Bise T, Baier F, Sallin P, Jaźwińska A. 2016. Preconditioning boosts regenerative programmes in the adult zebrafish heart. Open Biol. 6, 160101 (doi:10.1098/rsob.160101) - DOI - PMC - PubMed
-
- Heusch G. 2015. Molecular basis of cardioprotection: signal transduction in ischemic pre-, post-, and remote conditioning. Circ. Res. 116, 674–699. (doi:10.1161/CIRCRESAHA.116.305348) - DOI - PubMed
-
- Kalogeris T, Baines CP, Krenz M, Korthuis RJ. 2012. Cell biology of ischemia/reperfusion injury. Int. Rev. Cell Mol. Biol. 298, 229–317. (doi:10.1016/B978-0-12-394309-5.00006-7) - DOI - PMC - PubMed
-
- Dickson EW, Reinhardt CP, Renzi FP, Becker RC, Porcaro WA, Heard SO. 1999. Ischemic preconditioning may be transferable via whole blood transfusion: preliminary evidence. J. Thromb. Thrombolysis 8, 123–129. (doi:10.1023/A:1008911101951) - DOI - PubMed
-
- Breivik L, Helgeland E, Aarnes EK, Mrdalj J, Jonassen AK. 2011. Remote postconditioning by humoral factors in effluent from ischemic preconditioned rat hearts is mediated via PI3K/Akt-dependent cell-survival signaling at reperfusion. Basic Res. Cardiol. 106, 135–145. (doi:10.1007/s00395-010-0133-0) - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

