High glucose promotes the migration of retinal pigment epithelial cells through increased oxidative stress and PEDF expression
- PMID: 27440660
- PMCID: PMC5129761
- DOI: 10.1152/ajpcell.00001.2016
High glucose promotes the migration of retinal pigment epithelial cells through increased oxidative stress and PEDF expression
Abstract
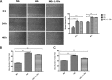
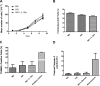
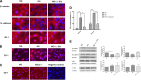
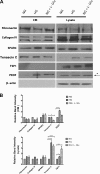
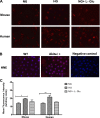
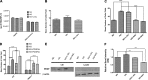
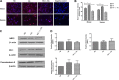
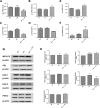
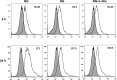
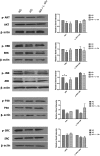
Defects in the outer blood-retinal barrier have significant impact on the pathogenesis of diabetic retinopathy and macular edema. However, the detailed mechanisms involved remain largely unknown. This is, in part, attributed to the lack of suitable animal and cell culture models, including those of mouse origin. We recently reported a method for the culture of retinal pigment epithelial (RPE) cells from wild-type and transgenic mice. The RPE cells are responsible for maintaining the integrity of the outer blood-retinal barrier whose dysfunction during diabetes has a significant impact on vision. Here we determined the impact of high glucose on the function of RPE cells. We showed that high glucose conditions resulted in enhanced migration and increased the level of oxidative stress in RPE cells, but minimally impacted their rate of proliferation and apoptosis. High glucose also minimally affected the cell-matrix and cell-cell interactions of RPE cells. However, the expression of integrins and extracellular matrix proteins including pigment epithelium-derived factor (PEDF) were altered under high glucose conditions. Incubation of RPE cells with the antioxidant N-acetylcysteine under high glucose conditions restored normal migration and PEDF expression. These cells also exhibited increased nuclear localization of the antioxidant transcription factor Nrf2 and ZO-1, reduced levels of β-catenin and phagocytic activity, and minimal effect on production of vascular endothelial growth factor, inflammatory cytokines, and Akt, MAPK, and Src signaling pathways. Thus high glucose conditions promote RPE cell migration through increased oxidative stress and expression of PEDF without a significant effect on the rate of proliferation and apoptosis.
Keywords: apoptosis; cell signaling; diabetes; diabetic retinopathy; outer retinal barrier.
Copyright © 2016 the American Physiological Society.
Figures



Similar articles
-
PEDF protects human retinal pigment epithelial cells against oxidative stress via upregulation of UCP2 expression.Mol Med Rep. 2019 Jan;19(1):59-74. doi: 10.3892/mmr.2018.9645. Epub 2018 Nov 12. Mol Med Rep. 2019. PMID: 30431098 Free PMC article.
-
Induction and Analysis of Oxidative Stress in Sleeping Beauty Transposon-Transfected Human Retinal Pigment Epithelial Cells.J Vis Exp. 2020 Dec 11;(166). doi: 10.3791/61957. J Vis Exp. 2020. PMID: 33369607
-
[The correlation between the concentrations of VEGF and PEDF and Ca2+-PKC signaling pathways in human retinal pigment epithelial cells cultured in vitro after exposuring to blue light].Zhonghua Yan Ke Za Zhi. 2015 Nov;51(11):839-43. Zhonghua Yan Ke Za Zhi. 2015. PMID: 26850586 Chinese.
-
[Role of the retinal pigment epithelium (RPE) in the pathogenesis and treatment of diabetic macular edema (DME)].Klin Oczna. 2008;110(7-9):318-20. Klin Oczna. 2008. PMID: 19112870 Review. Polish.
-
Mechanisms of PEDF-mediated protection against reactive oxygen species damage in diabetic retinopathy and neuropathy.J Endocrinol. 2014 Sep;222(3):R129-39. doi: 10.1530/JOE-14-0065. Epub 2014 Jun 13. J Endocrinol. 2014. PMID: 24928938 Review.
Cited by
-
α1D Adrenergic Receptor Antagonism Protects Against High Glucose-Induced Mitochondrial Dysfunction and Blood Retinal Barrier Breakdown in ARPE-19 Cells.Int J Mol Sci. 2025 Jan 24;26(3):967. doi: 10.3390/ijms26030967. Int J Mol Sci. 2025. PMID: 39940746 Free PMC article.
-
Scutellaria barbata D. Don Polysaccharides Inhibit High Glucose-Induced Proliferation and Angiogenesis of Retinal Vascular Endothelial Cells.Diabetes Metab Syndr Obes. 2021 May 31;14:2431-2440. doi: 10.2147/DMSO.S296164. eCollection 2021. Diabetes Metab Syndr Obes. 2021. PMID: 34103952 Free PMC article.
-
TIN2 modulates FOXO1 mitochondrial shuttling to enhance oxidative stress-induced apoptosis in retinal pigment epithelium under hyperglycemia.Cell Death Differ. 2024 Nov;31(11):1487-1505. doi: 10.1038/s41418-024-01349-8. Epub 2024 Jul 30. Cell Death Differ. 2024. PMID: 39080375
-
Factors affecting the response to subthreshold micropulse laser therapy used in center-involved diabetic macular edema.Lasers Med Sci. 2022 Apr;37(3):1865-1871. doi: 10.1007/s10103-021-03441-8. Epub 2021 Oct 17. Lasers Med Sci. 2022. PMID: 34657215
-
Astragalus polysaccharides suppresses high glucose-induced metabolic memory in retinal pigment epithelial cells through inhibiting mitochondrial dysfunction-induced apoptosis by regulating miR-195.Mol Med. 2019 May 22;25(1):21. doi: 10.1186/s10020-019-0088-z. Mol Med. 2019. PMID: 31117931 Free PMC article.
References
-
- Abcouwer SF, Antonetti DA. A role for systemic inflammation in diabetic retinopathy. Invest Ophthalmol Vis Sci 54: 2384, 2013. - PubMed
-
- Aiello LP. Angiogenic pathways in diabetic retinopathy. N Engl J Med 353: 839–841, 2005. - PubMed
-
- Al-Shabrawey M, Mussell R, Kahook K, Tawfik A, Eladl M, Sarthy V, Nussbaum J, El-Marakby A, Park SY, Gurel Z, Sheibani N, Maddipati KR. Increased expression and activity of 12-lipoxygenase in oxygen-induced ischemic retinopathy and proliferative diabetic retinopathy: implications in retinal neovascularization. Diabetes 60: 614–624, 2011. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

