A neo-W chromosome in a tropical butterfly links colour pattern, male-killing, and speciation
- PMID: 27440667
- PMCID: PMC4971206
- DOI: 10.1098/rspb.2016.0821
A neo-W chromosome in a tropical butterfly links colour pattern, male-killing, and speciation
Abstract
Sexually antagonistic selection can drive both the evolution of sex chromosomes and speciation itself. The tropical butterfly the African Queen, Danaus chrysippus, shows two such sexually antagonistic phenotypes, the first being sex-linked colour pattern, the second, susceptibility to a male-killing, maternally inherited mollicute, Spiroplasma ixodeti, which causes approximately 100% mortality in male eggs and first instar larvae. Importantly, this mortality is not affected by the infection status of the male parent and the horizontal transmission of Spiroplasma is unknown. In East Africa, male-killing of the Queen is prevalent in a narrow hybrid zone centred on Nairobi. This hybrid zone separates otherwise allopatric subspecies with different colour patterns. Here we show that a neo-W chromosome, a fusion between the W (female) chromosome and an autosome that controls both colour pattern and male-killing, links the two phenotypes thereby driving speciation across the hybrid zone. Studies of the population genetics of the neo-W around Nairobi show that the interaction between colour pattern and male-killer susceptibility restricts gene flow between two subspecies of D. chrysippus Our results demonstrate how a complex interplay between sex, colour pattern, male-killing, and a neo-W chromosome, has set up a genetic 'sink' that keeps the two subspecies apart. The association between the neo-W and male-killing thus provides a 'smoking gun' for an ongoing speciation process.
Keywords: Danaus chrysippus; Spiroplasma; colour pattern; male-killing; neo-W chromosome; speciation.
© 2016 The Authors.
Figures


 trivalent is found only in the mutant females c and e. Chromosomes in square brackets in females c, e, and male f are lost in successive generations of dead sons (marked with a cross in f). A few f males escape death by either immunity (MK suppression) or failed transmission of Spiroplasma [8]; most male survivors have the transiens (Cc) phenotype detectable by the white spots (arrowed in e,f) on the underside and/or scattered black scales on the upper side of the forewing apex.
trivalent is found only in the mutant females c and e. Chromosomes in square brackets in females c, e, and male f are lost in successive generations of dead sons (marked with a cross in f). A few f males escape death by either immunity (MK suppression) or failed transmission of Spiroplasma [8]; most male survivors have the transiens (Cc) phenotype detectable by the white spots (arrowed in e,f) on the underside and/or scattered black scales on the upper side of the forewing apex.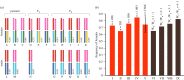
References
-
- Hurst GDD, Majerus MEN. 1993. Why do maternally inherited microorganisms kill males. Heredity 71, 81–95. (10.1038/hdy.1993.110) - DOI
-
- Owen DF, Chanter DO. 1968. Population of tropical African butterflies. 2. Sex ratio and polymorphism in Danaus chrysippus L. Rev. Zool. Bot. Afr. 78, 81–97.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
