Lost opportunity to save newborn lives: variable national antenatal screening policies for Neisseria gonorrhoeae and Chlamydia trachomatis
- PMID: 27440873
- PMCID: PMC6879101
- DOI: 10.1177/0956462416660483
Lost opportunity to save newborn lives: variable national antenatal screening policies for Neisseria gonorrhoeae and Chlamydia trachomatis
Abstract
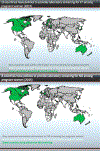
Unfavorable pregnancy outcomes caused by Chlamydia trachomatis or Neisseria gonorrhoeae infection are well known. The first step in addressing antenatal C. trachomatis and N. gonorrhoeae infection is a national policy to screen all pregnant women for C. trachomatis and N. gonorrhoeae, regardless of symptoms. The aim of this study was to inform policy makers on the presence of antenatal screening recommendations for C. trachomatis and N. gonorrhoeae infection. We conducted a three-part study from June 2015 to February 2016. We analyzed English and French language information online on Ministry of Health websites regarding C. trachomatis and N. gonorrhoeae antenatal screening. We referenced both primary official country and regional policy documents. We contacted the Ministry of Health directly if the information on the national antenatal screening was outdated or unavailable. In parallel, we sent a survey to the regional representative from the World Health Organization to help collect country-level data. Fourteen countries have current policies for antenatal screening of C. trachomatis and/or N. gonorrhoeae infection: Australia, the Bahamas, Bulgaria, Canada, Estonia, Japan, Germany, Latvia, New Zealand, Democratic People's Republic of Korea, Romania, Sweden, the United Kingdom, and the United States. Australia, New Zealand, and Latvia and the United States restricted antenatal screening to women ≤25 years old and those of higher risk. Several countries responded that they had policies to treat pregnant women with symptoms. This is the currently recommended WHO guideline but is not the same as universal screening. North Korea had policies in place which were not implemented due to lack of personnel and/or supplies. National level policies to support routine screening for C. trachomatis and N. gonorrhoeae infection to prevent adverse pregnancy and newborn outcomes are uncommon.
Keywords: Chlamydia (Chlamydia trachomatis); gonorrhea (Neisseria gonorrhoeae); prevention; screening; women.
Figures
Similar articles
-
Effect of antenatal Chlamydia trachomatis and Neisseria gonorrhoeae screening on postdelivery prevalence and vertical transmission in Gaborone, Botswana: findings from an exploratory study.Sex Transm Infect. 2025 Mar 24;101(2):81-87. doi: 10.1136/sextrans-2023-055965. Sex Transm Infect. 2025. PMID: 39366745 Clinical Trial.
-
Screening for Chlamydia trachomatis, Neisseria gonorrhoeae, and Mycoplasma genitalium should it be integrated into routine pregnancy care in French young pregnant women?Diagn Microbiol Infect Dis. 2015 May;82(1):14-9. doi: 10.1016/j.diagmicrobio.2015.01.014. Epub 2015 Feb 3. Diagn Microbiol Infect Dis. 2015. PMID: 25753079
-
Prevalence of Chlamydia trachomatis and Neisseria gonorrhoeae and repeat infection among pregnant urban adolescents.Sex Transm Dis. 2011 Mar;38(3):172-4. doi: 10.1097/OLQ.0b013e3181f41b96. Sex Transm Dis. 2011. PMID: 20938375 Free PMC article.
-
Molecular genetic methods for diagnosis and characterisation of Chlamydia trachomatis and Neisseria gonorrhoeae: impact on epidemiological surveillance and interventions.APMIS. 2004 Nov-Dec;112(11-12):771-84. doi: 10.1111/j.1600-0463.2004.apm11211-1205.x. APMIS. 2004. PMID: 15638837 Review.
-
Genital Chlamydia trachomatis and Neisseria gonorrhoeae infections among women in sub-Saharan Africa: A structured review.Int J STD AIDS. 2018 Jul;29(8):806-824. doi: 10.1177/0956462418758224. Epub 2018 Feb 28. Int J STD AIDS. 2018. PMID: 29486628 Review.
Cited by
-
Acceptability and Feasibility of Rapid Chlamydial, Gonococcal, and Trichomonal Screening and Treatment in Pregnant Women in 6 Low- to Middle-Income Countries.Sex Transm Dis. 2018 Oct;45(10):673-676. doi: 10.1097/OLQ.0000000000000832. Sex Transm Dis. 2018. PMID: 29528996 Free PMC article.
-
Neisseria gonorrhoeae Limits Chlamydia trachomatis Inclusion Development and Infectivity in a Novel In Vitro Co-Infection Model.Front Cell Infect Microbiol. 2022 Jul 7;12:911818. doi: 10.3389/fcimb.2022.911818. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35873141 Free PMC article.
-
Bridging the Gap Between Pilot and Scale-Up: A Model of Antenatal Testing for Curable Sexually Transmitted Infections From Botswana.Sex Transm Dis. 2022 Jan 1;49(1):59-66. doi: 10.1097/OLQ.0000000000001517. Sex Transm Dis. 2022. PMID: 34310524 Free PMC article.
-
Identifying the Impact of Chlamydia trachomatis Screening and Treatment on Mother-to-Child Transmission, and Respiratory Neonatal Outcomes in Mexico.Pathogens. 2024 Sep 28;13(10):843. doi: 10.3390/pathogens13100843. Pathogens. 2024. PMID: 39452715 Free PMC article.
-
Evaluating Chlamydia trachomatis and Neisseria gonorrhoeae screening and treatment among asymptomatic pregnant women to prevent preterm birth and low birthweight in Gaborone, Botswana: A secondary analysis from a non-randomised, cluster-controlled trial.BJOG. 2024 Aug;131(9):1259-1269. doi: 10.1111/1471-0528.17775. Epub 2024 Feb 13. BJOG. 2024. PMID: 38351649 Free PMC article. Clinical Trial.
References
-
- World Health Organization. [December 2015]; [February 16, 2016]; Sexually Transmitted Infections (STIs) Fact Sheet. Updated. Retrieved. from http://www.who.int/mediacentre/factsheets/fs110/en/
-
- Peuchant O, et al. (2015). “Screening for Chlamydia trachomatis, Neisseria gonorrhoeae, and Mycoplasma genitalium should it be integrated into routine pregnancy care in French young pregnant women?” Diagn Microbiol Infect Dis 82(1): 14–19. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical