Involvement of endocannabinoid neurotransmission in the bed nucleus of stria terminalis in cardiovascular responses to acute restraint stress in rats
- PMID: 27441413
- PMCID: PMC5055136
- DOI: 10.1111/bph.13560
Involvement of endocannabinoid neurotransmission in the bed nucleus of stria terminalis in cardiovascular responses to acute restraint stress in rats
Abstract
Background and purpose: Endocannabinoid signalling has been reported as an important neurochemical mechanism involved in responses to stress. Previous studies provided evidence of endocannabinoid release in the bed nucleus of the stria terminalis (BNST) during aversive stimuli. Nevertheless, a possible involvement of this neurochemical mechanism in stress responses has never been evaluated. Therefore, in the present study we investigated the involvement of BNST endocannabinoid neurotransmission, acting via local CB1 receptors, in the cardiovascular responses to acute restraint stress in rats.
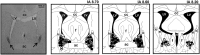
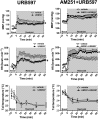
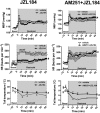
Experimental approach: The selective CB1 receptor antagonist AM251 (1, 30 and 100 pmol 100 nL(-1) ) and/or the fatty acid amide hydrolase (FAAH) enzyme inhibitor URB597 (30 pmol 100 nL(-1) ) or the monoacylglycerol lipase (MAGL) enzyme inhibitor JZL184 (30 pmol 100 nL(-1) ) was microinjected into the BNST before the acute restraint stress.
Key results: Microinjection of AM251 into the BNST enhanced the tachycardia caused by restraint stress, without affecting the increase in arterial pressure and the sympathetic-mediated cutaneous vasoconstrictor response. Conversely, the increased endogenous levels of AEA in the BNST evoked by local treatment with the FAAH enzyme inhibitor URB597 decreased restraint-evoked tachycardia. Inhibition of the hydrolysis of 2-arachidonoylglycerol (2-AG) in the BNST by local microinjection of the MAGL enzyme inhibitor JZL184 also decreased the HR response. These effects of URB597 and JZL184 were abolished by BNST pretreatment with AM251.
Conclusions and implications: These findings indicate an involvement of BNST endocannabinoid neurotransmission, acting via CB1 receptors, in cardiovascular adjustments during emotional stress, which may be mediated by the local release of either AEA or 2-AG.
© 2016 The British Pharmacological Society.
Figures

References
-
- Adhikari A (2014). Distributed circuits underlying anxiety. Front Behav Neurosci 8: 112. doi: 10.3389/fnbeh.2014.00112. - DOI - PMC - PubMed
-
- Bisogno T, Ligresti A, Di Marzo V (2005). The endocannabinoid signalling system: biochemical aspects. Pharmacol Biochem Behav 81: 224–238. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

