Genetic progression of Barrett's oesophagus to oesophageal adenocarcinoma
- PMID: 27441494
- PMCID: PMC4985359
- DOI: 10.1038/bjc.2016.219
Genetic progression of Barrett's oesophagus to oesophageal adenocarcinoma
Abstract
Barrett's oesophagus (BE) is the premalignant condition associated with the development of oesophageal adenocarcinoma (OAC). Diagnostically, p53 immunohistochemistry remains the only biomarker recommended clinically to aid histopathological diagnosis. The emerging mutational profile of BE is one of highly heterogeneous lesions at the genomic level with many mutations already occurring in non-dysplastic tissue. As well as point mutations, larger scale copy-number changes appear to have a key role in the progression to OAC and clinically applicable assays for the reliable detection of aneuploidy will be important to incorporate into future clinical management of patients. For some patients, the transition to malignancy may occur rapidly through a genome-doubling event or chromosomal catastrophe, termed chromothripsis, and detecting these patients may prove especially difficult. Given the heterogeneous nature of this disease, sampling methods to overcome inherent bias from endoscopic biopsies coupled with the development of more objective biomarkers than the current reliance on histopathology will be required for risk stratification. The aim of this approach will be to spare low-risk patients unnecessary procedures, as well as to provide endoscopic therapy to the patients at highest risk, thereby avoiding the burden of incurable metastatic disease.
Conflict of interest statement
RCF developed the Cytosponge technology and is named on related patents. This technology has been licensed by the MRC to Covidien GI Solutions, now Medtronic. The remaining authors declare no conflict of interest.
Figures
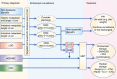

References
-
- Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, Bignell GR, Bolli N, Borg A, Børresen-Dale AL, Boyault S, Burkhardt B, Butler AP, Caldas C, Davies HR, Desmedt C, Eils R, Eyfjörd JE, Foekens JA, Greaves M, Hosoda F, Hutter B, Ilicic T, Imbeaud S, Imielinski M, Jäger N, Jones DT, Jones D, Knappskog S, Kool M, Lakhani SR, López-Otín C, Martin S, Munshi NC, Nakamura H, Northcott PA, Pajic M, Papaemmanuil E, Paradiso A, Pearson JV, Puente XS, Raine K, Ramakrishna M, Richardson AL, Richter J, Rosenstiel P, Schlesner M, Schumacher TN, Span PN, Teague JW, Totoki Y, Tutt AN, Valdés-Mas R, van Buuren MM, van't Veer L, Vincent-Salomon A, Waddell N, Yates LR. Australian Pancreatic Cancer Genome Initiative; ICGC Breast Cancer Consortium; ICGC MMML-Seq Consortium; ICGC PedBrain, Zucman-Rossi J, Futreal PA, McDermott U, Lichter P, Meyerson M, Grimmond SM, Siebert R, Campo E, Shibata T, Pfister SM, Campbell PJ, Stratton MR (2013) Signatures of mutational processes in human cancer. Nature 500: 415–421. - PMC - PubMed
-
- Alvi MA, Liu X, O'donovan M, Newton R, Wernisch L, Shannon NB, Shariff K, di Pietro M, Bergman JJ, Ragunath K, Fitzgerald RC (2013) DNA methylation as an adjunct to histopathology to detect prevalent, inconspicuous dysplasia and early-stage neoplasia in Barrett's esophagus. Clin Cancer Res 19: 878–888. - PMC - PubMed
-
- Bird-Lieberman EL, Dunn JM, Coleman HG, Lao-Sirieix P, Oukrif D, Moore CE, Varghese S, Johnston BT, Arthur K, McManus DT, Novelli MR, O'Donovan M, Cardwell CR, Lovat LB, Murray LJ, Fitzgerald RC (2012. a) Population-based study reveals new risk-stratification biomarker panel for Barrett's esophagus. Gastroenterology 143: 927–35 e3. - PubMed
-
- Bird-Lieberman EL, Neves AA, Lao-Sirieix P, O'donovan M, Novelli M, Lovat LB, Eng WS, Mahal LK, Brindle KM, Fitzgerald RC (2012. b) Molecular imaging using fluorescent lectins permits rapid endoscopic identification of dysplasia in Barrett's esophagus. Nat Med 18: 315–321. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

