Superior therapeutic efficacy of nab-paclitaxel over cremophor-based paclitaxel in locally advanced and metastatic models of human pancreatic cancer
- PMID: 27441498
- PMCID: PMC4985357
- DOI: 10.1038/bjc.2016.215
Superior therapeutic efficacy of nab-paclitaxel over cremophor-based paclitaxel in locally advanced and metastatic models of human pancreatic cancer
Abstract
Background: Albumin-bound paclitaxel (nab-paclitaxel, nab-PTX) plus gemcitabine (GEM) combination has demonstrated efficient antitumour activity and statistically significant overall survival of patients with metastatic pancreatic ductal adenocarcinoma (PDAC) compared with GEM monotherapy. This regimen is currently approved as a standard of care treatment option for patients with metastatic PDAC. It is unclear whether cremophor-based PTX combined with GEM provide a similar level of therapeutic efficacy in PDAC.
Methods: We comprehensively explored the antitumour efficacy, effect on metastatic dissemination, tumour stroma and survival advantage following GEM, PTX and nab-PTX as monotherapy or in combination with GEM in a locally advanced, and a highly metastatic orthotopic model of human PDAC.
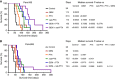
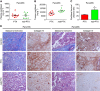
Results: Nab-PTX treatment resulted in significantly higher paclitaxel tumour plasma ratio (1.98-fold), robust stromal depletion, antitumour efficacy (3.79-fold) and survival benefit compared with PTX treatment. PTX plus GEM treatment showed no survival gain over GEM monotherapy. However, nab-PTX in combination with GEM decreased primary tumour burden, metastatic dissemination and significantly increased median survival of animals compared with either agents alone. These therapeutic effects were accompanied by depletion of dense fibrotic tumour stroma and decreased proliferation of carcinoma cells. Notably, nab-PTX monotherapy was equivalent to nab-PTX plus GEM in providing survival advantage to mice in a highly aggressive metastatic PDAC model, indicating that nab-PTX could potentially stop the progression of late-stage pancreatic cancer.
Conclusions: Our data confirmed that therapeutic efficacy of PTX and nab-PTX vary widely, and the contention that these agents elicit similar antitumour response was not supported. The addition of PTX to GEM showed no survival advantage, concluding that a clinical combination of PTX and GEM may unlikely to provide significant survival advantage over GEM monotherapy and may not be a viable alternative to the current standard-of-care nab-PTX plus GEM regimen for the treatment of PDAC patients.
Conflict of interest statement
ZT, SH, SB, DWP and CH are employees of Celgene Corporation. The remaining authors declare no conflict of interest.
Figures



Similar articles
-
Gemcitabine enhances the transport of nanovector-albumin-bound paclitaxel in gemcitabine-resistant pancreatic ductal adenocarcinoma.Cancer Lett. 2017 Sep 10;403:296-304. doi: 10.1016/j.canlet.2017.06.026. Epub 2017 Jul 4. Cancer Lett. 2017. PMID: 28687352 Free PMC article.
-
Altered Gemcitabine and Nab-paclitaxel Scheduling Improves Therapeutic Efficacy Compared with Standard Concurrent Treatment in Preclinical Models of Pancreatic Cancer.Clin Cancer Res. 2021 Jan 15;27(2):554-565. doi: 10.1158/1078-0432.CCR-20-1422. Epub 2020 Oct 21. Clin Cancer Res. 2021. PMID: 33087331 Free PMC article.
-
Preclinical Assessment with Clinical Validation of Selinexor with Gemcitabine and Nab-Paclitaxel for the Treatment of Pancreatic Ductal Adenocarcinoma.Clin Cancer Res. 2020 Mar 15;26(6):1338-1348. doi: 10.1158/1078-0432.CCR-19-1728. Epub 2019 Dec 12. Clin Cancer Res. 2020. PMID: 31831564 Free PMC article. Clinical Trial.
-
Nano albumin bound-paclitaxel in pancreatic cancer: Current evidences and future directions.World J Gastroenterol. 2017 Aug 28;23(32):5875-5886. doi: 10.3748/wjg.v23.i32.5875. World J Gastroenterol. 2017. PMID: 28932079 Free PMC article. Review.
-
Tumour-stroma interactions in pancreatic ductal adenocarcinoma: rationale and current evidence for new therapeutic strategies.Cancer Treat Rev. 2014 Feb;40(1):118-28. doi: 10.1016/j.ctrv.2013.04.004. Epub 2013 Jul 9. Cancer Treat Rev. 2014. PMID: 23849556 Review.
Cited by
-
Heme Oxygenase-1 Inhibition Potentiates the Effects of Nab-Paclitaxel-Gemcitabine and Modulates the Tumor Microenvironment in Pancreatic Ductal Adenocarcinoma.Cancers (Basel). 2021 May 8;13(9):2264. doi: 10.3390/cancers13092264. Cancers (Basel). 2021. PMID: 34066839 Free PMC article.
-
The Role of Microtubules in Pancreatic Cancer: Therapeutic Progress.Front Oncol. 2021 May 21;11:640863. doi: 10.3389/fonc.2021.640863. eCollection 2021. Front Oncol. 2021. PMID: 34094924 Free PMC article. Review.
-
Nanocarriers for pancreatic cancer imaging, treatments, and immunotherapies.Theranostics. 2022 Jan 1;12(3):1030-1060. doi: 10.7150/thno.64805. eCollection 2022. Theranostics. 2022. PMID: 35154473 Free PMC article. Review.
-
Optimization and Development of Selective Histone Deacetylase Inhibitor (MPT0B291)-Loaded Albumin Nanoparticles for Anticancer Therapy.Pharmaceutics. 2021 Oct 19;13(10):1728. doi: 10.3390/pharmaceutics13101728. Pharmaceutics. 2021. PMID: 34684020 Free PMC article.
-
Clinical efficacy of nab-paclitaxel in patients with metastatic pancreatic cancer.Drug Des Devel Ther. 2018 Jun 19;12:1769-1775. doi: 10.2147/DDDT.S165851. eCollection 2018. Drug Des Devel Ther. 2018. PMID: 29950811 Free PMC article.
References
-
- Alvarez R, Musteanu M, Garcia-Garcia E, Lopez-Casas PP, Megias D, Guerra C, Munoz M, Quijano Y, Cubillo A, Rodriguez-Pascual J, Plaza C, de Vicente E, Prados S, Tabernero S, Barbacid M, Lopez-Rios F, Hidalgo M (2013) Stromal disrupting effects of nab-paclitaxel in pancreatic cancer. Br J Cancer 109(4): 926–933. - PMC - PubMed
-
- Biankin AV, Waddell N, Kassahn KS, Gingras MC, Muthuswamy LB, Johns AL, Miller DK, Wilson PJ, Patch AM, Wu J, Chang DK, Cowley MJ, Gardiner BB, Song S, Harliwong I, Idrisoglu S, Nourse C, Nourbakhsh E, Manning S, Wani S, Gongora M, Pajic M, Scarlett CJ, Gill AJ, Pinho AV, Rooman I, Anderson M, Holmes O, Leonard C, Taylor D, Wood S, Xu Q, Nones K, Fink JL, Christ A, Bruxner T, Cloonan N, Kolle G, Newell F, Pinese M, Mead RS, Humphris JL, Kaplan W, Jones MD, Colvin EK, Nagrial AM, Humphrey ES, Chou A, Chin VT, Chantrill LA, Mawson A, Samra JS, Kench JG, Lovell JA, Daly RJ, Merrett ND, Toon C, Epari K, Nguyen NQ, Barbour A, Zeps N. Australian Pancreatic Cancer Genome I Kakkar N, Zhao F, Wu YQ, Wang M, Muzny DM, Fisher WE, Brunicardi FC, Hodges SE, Reid JG, Drummond J, Chang K, Han Y, Lewis LR, Dinh H, Buhay CJ, Beck T, Timms L, Sam M, Begley K, Brown A, Pai D, Panchal A, Buchner N, De Borja R, Denroche RE, Yung CK, Serra S, Onetto N, Mukhopadhyay D, Tsao MS, Shaw PA, Petersen GM, Gallinger S, Hruban RH, Maitra A, Iacobuzio-Donahue CA, Schulick RD, Wolfgang CL, Morgan RA, Lawlor RT, Capelli P, Corbo V, Scardoni M, Tortora G, Tempero MA, Mann KM, Jenkins NA, Perez-Mancera PA, Adams DJ, Largaespada DA, Wessels LF, Rust AG, Stein LD, Tuveson DA, Copeland NG, Musgrove EA, Scarpa A, Eshleman JR, Hudson TJ, Sutherland RL, Wheeler DA, Pearson JV, McPherson JD, Gibbs RA, Grimmond SM (2012) Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature 491(7424): 399–405. - PMC - PubMed
-
- Burris HA 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 15(6): 2403–2413. - PubMed
-
- Chiorean EG, Von Hoff DD (2014) Taxanes: impact on pancreatic cancer. Anticancer Drugs 25(5): 584–592. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

