Dynein Light Intermediate Chain 2 Facilitates the Metaphase to Anaphase Transition by Inactivating the Spindle Assembly Checkpoint
- PMID: 27441562
- PMCID: PMC4956306
- DOI: 10.1371/journal.pone.0159646
Dynein Light Intermediate Chain 2 Facilitates the Metaphase to Anaphase Transition by Inactivating the Spindle Assembly Checkpoint
Abstract
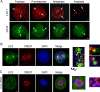
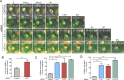
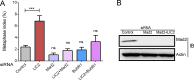
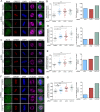
The multi-functional molecular motor cytoplasmic dynein performs diverse essential roles during mitosis. The mechanistic importance of the dynein Light Intermediate Chain homologs, LIC1 and LIC2 is unappreciated, especially in the context of mitosis. LIC1 and LIC2 are believed to exist in distinct cytoplasmic dynein complexes as obligate subunits. LIC1 had earlier been reported to be required for metaphase to anaphase progression by inactivating the kinetochore-microtubule attachment-sensing arm of the spindle assembly checkpoint (SAC). However, the functional importance of LIC2 during mitosis remains elusive. Here we report prominent novel roles for the LIC2 subunit of cytoplasmic dynein in regulating the spindle assembly checkpoint. LIC2 depletion in mammalian cells led to prolonged metaphase arrest in the presence of an active SAC and also to stretched kinetochores, thus implicating it in SAC inactivation. Quantitative fluorescence microscopy of SAC components revealed accumulation of both attachment- and tension-sensing checkpoint proteins at metaphase kinetochores upon LIC2 depletion. These observations support a stronger and more diverse role in checkpoint inactivation for LIC2 in comparison to its close homolog LIC1. Our study uncovers a novel functional hierarchy during mitotic checkpoint inactivation between the closely related but homologous LIC subunits of cytoplasmic dynein. These subtle functional distinctions between dynein subpopulations could be exploited to study specific aspects of the spindle assembly checkpoint, which is a key mediator of fidelity in eukaryotic cell division.
Conflict of interest statement
Figures



References
-
- Biggins S, Walczak CE. Captivating capture: how microtubules attach to kinetochores. Curr Biol. 2003;13(11):R449–60. Epub 2003/06/05. . - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

