Loss of Fertility in the Absence of Progesterone Receptor Expression in Kisspeptin Neurons of Female Mice
- PMID: 27441639
- PMCID: PMC4956300
- DOI: 10.1371/journal.pone.0159534
Loss of Fertility in the Absence of Progesterone Receptor Expression in Kisspeptin Neurons of Female Mice
Abstract
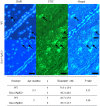
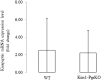
Ovarian steroids, estradiol and progesterone, play central roles in regulating female reproduction by acting as both positive and negative regulators of gonadotropin-releasing hormone (GnRH) secretion in the hypothalamus. Recent studies have identified kisspeptin neurons of the hypothalamus as the target of estrogenic regulation of GnRH secretion. In this study, we aimed to determine the significance of progesterone receptor (PGR) expression in the kisspeptin neurons. To this end, the Pgr gene was selectively ablated in mouse kisspeptin neurons and the reproductive consequence assessed. The hypothalamus of the Pgr deficient female mouse expressed kisspeptin, the pituitary released LH in response to GnRH stimulation, and the ovary ovulated when stimulated with gonadotropins. However, the mutant mouse gradually lost cyclicity, was unable to generate a LH surge in response to rising estradiol, and eventually became infertile. Taken together, these results indicate that the loss of PGR impairs kisspeptin secretory machinery and therefore that PGR plays a critical role in regulating kisspeptin secretion.
Conflict of interest statement
Figures
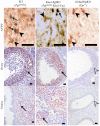
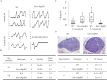
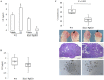
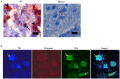
Similar articles
-
Progesterone Receptors in AVPV Kisspeptin Neurons Are Sufficient for Positive Feedback Induction of the LH Surge.Endocrinology. 2021 Nov 1;162(11):bqab161. doi: 10.1210/endocr/bqab161. Endocrinology. 2021. PMID: 34379733 Free PMC article.
-
Chronic estradiol exposure suppresses luteinizing hormone surge without affecting kisspeptin neurons and estrogen receptor alpha in anteroventral periventricular nucleus†.Biol Reprod. 2024 Jan 13;110(1):90-101. doi: 10.1093/biolre/ioad129. Biol Reprod. 2024. PMID: 37774351
-
Reduction of Kiss1 expression in the anteroventral periventricular nucleus is associated with atrazine-induced attenuation of the luteinizing hormone surge in female rats.Biol Reprod. 2019 Jan 1;100(1):41-48. doi: 10.1093/biolre/ioy159. Biol Reprod. 2019. PMID: 30010721
-
Hypothalamic Astrocyte Development and Physiology for Neuroprogesterone Induction of the Luteinizing Hormone Surge.Front Endocrinol (Lausanne). 2020 Jun 26;11:420. doi: 10.3389/fendo.2020.00420. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 32670203 Free PMC article. Review.
-
Minireview: kisspeptin neurons as central processors in the regulation of gonadotropin-releasing hormone secretion.Endocrinology. 2006 Mar;147(3):1154-8. doi: 10.1210/en.2005-1282. Epub 2005 Dec 22. Endocrinology. 2006. PMID: 16373418 Review.
Cited by
-
Hysteroscopic injections of autologous endometrial cells and platelet-rich plasma in patients with thin endometrium: a pilot randomized study.Sci Rep. 2023 Jan 18;13(1):945. doi: 10.1038/s41598-023-27982-w. Sci Rep. 2023. PMID: 36653431 Free PMC article. Clinical Trial.
-
Rodent Models of Non-classical Progesterone Action Regulating Ovulation.Front Endocrinol (Lausanne). 2017 Jul 24;8:165. doi: 10.3389/fendo.2017.00165. eCollection 2017. Front Endocrinol (Lausanne). 2017. PMID: 28790975 Free PMC article. Review.
-
Neuroendocrine mechanisms underlying estrogen positive feedback and the LH surge.Front Neurosci. 2022 Jul 27;16:953252. doi: 10.3389/fnins.2022.953252. eCollection 2022. Front Neurosci. 2022. PMID: 35968365 Free PMC article. Review.
-
Increased FOXL2 expression alters uterine structures and functions†.Biol Reprod. 2020 Oct 29;103(5):951-965. doi: 10.1093/biolre/ioaa143. Biol Reprod. 2020. PMID: 32948877 Free PMC article.
-
Localization of kisspeptin, NKB, and NK3R in the hypothalamus of gilts treated with the progestin altrenogest.Biol Reprod. 2021 Oct 11;105(4):1056-1067. doi: 10.1093/biolre/ioab103. Biol Reprod. 2021. PMID: 34037695 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

