Proton pump inhibitors protect mice from acute systemic inflammation and induce long-term cross-tolerance
- PMID: 27441656
- PMCID: PMC4973356
- DOI: 10.1038/cddis.2016.218
Proton pump inhibitors protect mice from acute systemic inflammation and induce long-term cross-tolerance
Abstract
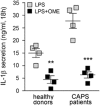
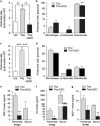
Incidence of sepsis is increasing, representing a tremendous burden for health-care systems. Death in acute sepsis is attributed to hyperinflammatory responses, but the underlying mechanisms are still unclear. We report here that proton pump inhibitors (PPIs), which block gastric acid secretion, selectively inhibited tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) secretion by Toll-like receptor (TLR)-activated human monocytes in vitro, in the absence of toxic effects. Remarkably, the oversecretion of IL-1β that represents a hallmark of monocytes from patients affected by cryopyrin-associated periodic syndrome is also blocked. Based on these propaedeutic experiments, we tested the effects of high doses of PPIs in vivo in the mouse model of endotoxic shock. Our data show that a single administration of PPI protected mice from death (60% survival versus 5% of untreated mice) and decreased TNF-α and IL-1β systemic production. PPIs were efficacious even when administered after lipopolysaccharide (LPS) injection. PPI-treated mice that survived developed a long-term cross-tolerance, becoming resistant to LPS- and zymosan-induced sepsis. In vitro, their macrophages displayed impaired TNF-α and IL-1β to different TLR ligands. PPIs also prevented sodium thioglycollate-induced peritoneal inflammation, indicating their efficacy also in a non-infectious setting independent of TLR stimulation. Lack of toxicity and therapeutic effectiveness make PPIs promising new drugs against sepsis and other severe inflammatory conditions.
Figures






References
-
- Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med 2003; 348: 138–150. - PubMed
-
- Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med 2013; 369: 840–851. - PubMed
-
- Lagu T, Rothberg MB, Shieh MS, Pekow PS, Steingrub JS, Lindenauer PK. Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med 2007; 40: 754–761. - PubMed
-
- Levy MM, Artigas A, Phillips GS, Rhodes A, Beale R, Osborn T et al. Outcomes of the surviving sepsis campaign in intensive care units in the USA and Europe: a prospective cohort study. Lancet Infect Dis 2012; 12: 919–924. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

