Generation and Characterization of a Bivalent HIV-1 Subtype C gp120 Protein Boost for Proof-of-Concept HIV Vaccine Efficacy Trials in Southern Africa
- PMID: 27442017
- PMCID: PMC4956256
- DOI: 10.1371/journal.pone.0157391
Generation and Characterization of a Bivalent HIV-1 Subtype C gp120 Protein Boost for Proof-of-Concept HIV Vaccine Efficacy Trials in Southern Africa
Abstract
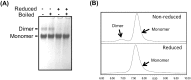
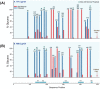
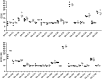
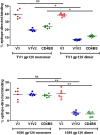
The viral envelope glycoprotein (Env) is the major target for antibody (Ab)-mediated vaccine development against the Human Immunodeficiency Virus type 1 (HIV-1). Although several recombinant Env antigens have been evaluated in clinical trials, only the surface glycoprotein, gp120, (from HIV-1 subtype B, MN, and subtype CRF_01AE, A244) used in the ALVAC prime-AIDSVAX gp120 boost RV144 Phase III HIV vaccine trial was shown to contribute to protective efficacy, although modest and short-lived. Hence, for clinical trials in southern Africa, a bivalent protein boost of HIV-1 subtype C gp120 antigens composed of two complementary gp120s, from the TV1.C (chronic) and 1086.C (transmitted founder) HIV-1 strains, was selected. Stable Chinese Hamster Cell (CHO) cell lines expressing these gp120s were generated, scalable purification methods were developed, and a detailed analytical analysis of the purified proteins was conducted that showed differences and complementarity in the antigenicity, glycan occupancy, and glycan content of the two gp120 molecules. Moreover, mass spectrometry revealed some disulfide heterogeneity in the expressed proteins, particularly in V1V2-C1 region and most prominently in the TV1 gp120 dimers. These dimers not only lacked binding to certain key CD4 binding site (CD4bs) and V1V2 epitope-directed ligands but also elicited reduced Ab responses directed to those epitopes, in contrast to monomeric gp120, following immunization of rabbits. Both monomeric and dimeric gp120s elicited similarly high titer Tier 1 neutralizing Abs as measured in standard virus neutralization assays. These results provide support for clinical evaluations of bivalent preparations of purified monomeric TV1.C and 1086.C gp120 proteins.
Conflict of interest statement
Figures


References
-
- Andrews S. Access to essential medications for HIV/AIDS in South Africa. South African medical journal = Suid-Afrikaanse tydskrif vir geneeskunde. 2001;91(5):384–7. . - PubMed
-
- Bogard E, Kuntz KM. The impact of a partially effective HIV vaccine on a population of intravenous drug users in Bangkok, Thailand: a dynamic model. Journal of acquired immune deficiency syndromes. 2002;29(2):132–41. . - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

