ARGOS8 variants generated by CRISPR-Cas9 improve maize grain yield under field drought stress conditions
- PMID: 27442592
- PMCID: PMC5258859
- DOI: 10.1111/pbi.12603
ARGOS8 variants generated by CRISPR-Cas9 improve maize grain yield under field drought stress conditions
Abstract
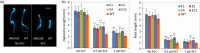
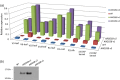
Maize ARGOS8 is a negative regulator of ethylene responses. A previous study has shown that transgenic plants constitutively overexpressing ARGOS8 have reduced ethylene sensitivity and improved grain yield under drought stress conditions. To explore the targeted use of ARGOS8 native expression variation in drought-tolerant breeding, a diverse set of over 400 maize inbreds was examined for ARGOS8 mRNA expression, but the expression levels in all lines were less than that created in the original ARGOS8 transgenic events. We then employed a CRISPR-Cas-enabled advanced breeding technology to generate novel variants of ARGOS8. The native maize GOS2 promoter, which confers a moderate level of constitutive expression, was inserted into the 5'-untranslated region of the native ARGOS8 gene or was used to replace the native promoter of ARGOS8. Precise genomic DNA modification at the ARGOS8 locus was verified by PCR and sequencing. The ARGOS8 variants had elevated levels of ARGOS8 transcripts relative to the native allele and these transcripts were detectable in all the tissues tested, which was the expected results using the GOS2 promoter. A field study showed that compared to the WT, the ARGOS8 variants increased grain yield by five bushels per acre under flowering stress conditions and had no yield loss under well-watered conditions. These results demonstrate the utility of the CRISPR-Cas9 system in generating novel allelic variation for breeding drought-tolerant crops.
Keywords: ARGOS; CRISPR-Cas9; drought tolerance; genome editing; grain yield; maize.
© 2016 The Authors. Plant Biotechnology Journal published by Society for Experimental Biology and The Association of Applied Biologists and John Wiley & Sons Ltd.
Conflict of interest statement
The authors are employees of DuPont Pioneer.
Figures


References
-
- Ananiev, E.V. , Wu, C. , Chamberlin, M.A. , Svitashev, S. , Schwartz, C. , Gordon‐Kamm, W. and Tingey, S. (2009) Artificial chromosome formation in maize (Zea mays L.). Chromosoma, 118, 157–177. - PubMed
-
- Barbour, E. , Meyer, T.E. and Saad, M.E. (2003) Maize GOS2 Promoters. U.S. Patent No. 6504083 B1, Pioneer Hi‐Bred International.
-
- Bleecker, A.B. , Estelle, M.A. , Somerville, C. and Kende, H. (1988) Insensitivity to ethylene conferred by a dominant mutation in Arabidopsis thaliana. Science, 241, 1086–1089. - PubMed
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

