The Molecular Landscape of Recurrent and Metastatic Head and Neck Cancers: Insights From a Precision Oncology Sequencing Platform
- PMID: 27442865
- PMCID: PMC5253129
- DOI: 10.1001/jamaoncol.2016.1790
The Molecular Landscape of Recurrent and Metastatic Head and Neck Cancers: Insights From a Precision Oncology Sequencing Platform
Abstract
Importance: Recurrent and/or metastatic head and neck cancer is usually incurable. Implementation of precision oncology for these patients has been limited by incomplete understanding of the molecular alterations underlying advanced disease. At the same time, the molecular profiles of many rare head and neck cancer types are unknown. These significant gaps in knowledge need to be addressed to rationally devise new therapies.
Objective: To illuminate the distinct biology of recurrent and metastatic head and neck cancers and review implementation of precision oncology for patients with advanced disease.
Design, setting, and participants: After exclusions, 151 patients with advanced, treatment-resistant head and neck tumors, including squamous cell carcinoma (HNSCC), adenoid cystic carcinoma (ACC), and other salivary and cutaneous cancers, whose tumors were sequenced between January 2014 and July 2015 at Memorial Sloan Kettering were recruited. Next-generation sequencing of tumors as part of clinical care included high-depth (median 600×) exonic coverage of 410 cancer genes and whole-genome copy number analysis.
Interventions: Next-generation sequencing of tumors and matched normal DNA.
Main outcomes and measures: Feasibility, the frequency of actionable molecular alterations, the effect on decision making, and identification of alterations associated with recurrent and metastatic disease.
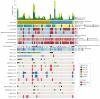
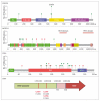
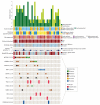
Results: Overall, 151 patients (95 men and 56 women; mean [range] age, 61.8 [17-100] years) were included in the study. Next-generation sequencing ultimately guided therapy in 21 of 151 patients (14%) (13 of 53 [25%] of patients with HNSCC) by refining diagnoses and matching patients to specific therapies, in some cases with dramatic responses on basket studies. Molecular alterations were potentially actionable in 28 of 135 patients (21%). The genetic profiles of recurrent and metastatic tumors were often distinct from primary tumors. Compared to primary human papillomavirus (HPV)-positive tumors, many recurrent and metastatic HPV-positive tumors exhibited a molecular profile more similar to HPV-negative tumors, including enriched frequencies of TP53 mutation (3 of 20 tumors [15%]), whole genome duplication (5 of 20 tumors [25%]), and 3p deletion (11 of 20 tumors [55%]). There were high rates of TERT promoter mutation in recurrent and metastatic HPV-negative HNSCC (13 of 30 tumors [43%]), cutaneous SCC (11 of 21 tumors [52%]), basal cell carcinoma (3 of 4 tumors [75%]), and ACC (5 of 36 tumors [14%]). Activating NOTCH1 mutations were enriched in metastatic ACCs (8 of 36 tumors [22%]).
Conclusions and relevance: These findings reveal the molecular landscape of advanced disease and rare cancer subtypes, both predominant challenges in head and neck oncology. To understand the repertoire of targetable alterations in advanced cancers, it is necessary to sequence recurrent and metastatic tumors. These data are important first steps toward implementation of precision head and neck oncology.
Figures




References
-
- Duvvuri U, Myers JN. Cancer of the head and neck is the sixth most common cancer worldwide. Curr Probl Surg. 2009;46(2):114–117. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

