Advances in understanding the mechanisms of erythropoiesis in homeostasis and disease
- PMID: 27442953
- PMCID: PMC6204224
- DOI: 10.1111/bjh.14194
Advances in understanding the mechanisms of erythropoiesis in homeostasis and disease
Abstract
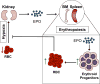
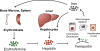
Anaemia or decreased blood haemoglobin is the most common blood disorder often characterized by reduced red blood cell (RBC) numbers. RBCs are produced from differentiation and commitment of haematopoietic stem cells to the erythroid lineage by a process called erythropoiesis. Coordination of erythropoietin receptor signalling with several erythroid transcription factors including GATA1 is essential for this process. A number of additional players that are critical for RBC production have been identified in recent years. Major technological advances, such as the development of RNA interference, genetically modified animals, including zebrafish, and imaging flow cytometry have led to these discoveries; the emergence of -omics approaches in combination with the optimization of ex vivo erythroid cultures have also produced a more comprehensive understanding of erythropoiesis. Here we summarize studies describing novel regulators of erythropoiesis that modulate erythroid cell production in the context of human erythroid disorders involving hypoxia, iron regulation, immune-related molecules, and the transcription factor FOXO3.
Keywords: erythropoiesis; homeostasis; mechanisms.
© 2016 John Wiley & Sons Ltd.
Figures



References
-
- Adolfsson J, Månsson R, Buza-Vidas N, Hultquist A, Liuba K, Jensen CT, Bryder D, Yang L, Borge OJ, Thoren LAM, Anderson K, Sitnicka E, Sasaki Y, Sigvardsson M, Jacobsen SEW. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell. 2005;121:295–306. - PubMed
-
- Anderson ER, Taylor M, Xue X, Ramakrishnan SK, Martin A, Xie L, Bredell BX, Gardenghi S, Rivella S, Shah YM. Intestinal HIF2α promotes tissue-iron accumulation in disorders of iron overload with anemia. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E4922–30. - PMC - PubMed
-
- Arlet JB, Ribeil JA, Guillem F, Negre O, Hazoume A, Marcion G, Beuzard Y, Dussiot M, Moura IC, Demarest S, de Beauchêne IC, Belaid-Choucair Z, Sevin M, Maciel TT, Auclair C, Leboulch P, Chretien S, Tchertanov L, Baudin-Creuza V, Seigneuric R, Fontenay M, Garrido C, Hermine O, Courois G. HSP70 sequestration by free α-globin promotes ineffective erythropoiesis in β-thalassaemia. Nature. 2014;514:242–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

