Use of CA-125 Tests and Computed Tomographic Scans for Surveillance in Ovarian Cancer
- PMID: 27442965
- PMCID: PMC5106306
- DOI: 10.1001/jamaoncol.2016.1842
Use of CA-125 Tests and Computed Tomographic Scans for Surveillance in Ovarian Cancer
Abstract
Importance: A 2009 randomized clinical trial demonstrated that using cancer antigen 125 (CA-125) tests for routine surveillance in ovarian cancer increases the use of chemotherapy and decreases patients' quality of life without improving survival, compared with clinical observation. The Society of Gynecologic Oncology guidelines categorize CA-125 testing as optional and discourage the use of radiographic imaging for routine surveillance. To date, few studies have examined the use of CA-125 tests in clinical practice.
Objectives: To examine the use of CA-125 tests and computed tomographic (CT) scans in clinical practice before and after the 2009 randomized clinical trial and to estimate the economic effect of surveillance testing.
Design, setting, and participants: A prospective cohort of 1241 women with ovarian cancer in clinical remission after completion of primary cytoreductive surgery and chemotherapy at 6 National Cancer Institute-designated cancer centers between January 1, 2004, and December 31, 2011, was followed up through December 31, 2012, to study the use of CA-125 tests and CT scans before and after 2009. Data analysis was conducted from April 9, 2014, to March 28, 2016.
Main outcomes and measures: The use of CA-125 tests and CT scans before and after 2009. Secondary outcomes included the time from CA-125 markers doubling to retreatment among women who experienced a rise in CA-125 markers before and after 2009, and the costs associated with surveillance testing using 2015 Medicare reimbursement rates.
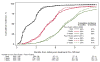
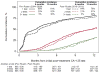
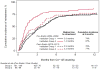
Results: Among 1241 women (mean [SD] age 59 [12] years; 1112 white [89.6%]), the use of CA-125 testing and CT scans was similar during the study period. During 12 months of surveillance, the cumulative incidence of patients undergoing 3 or more CA-125 tests was 86% in 2004-2009 vs 91% in 2010-2012 (P = .95), and the cumulative incidence of patients undergoing more than 1 CT scan was 81% in 2004-2009 vs 78% in 2010-2012 (P = .50). Among women whose CA-125 markers doubled (n = 511), there was no significant difference in the time to retreatment with chemotherapy before and after 2009 (median, 2.8 vs 3.5 months; P = .40). During a 12-month period, there was a mean of 4.6 CA-125 tests and 1.7 CT scans performed per patient, resulting in a US population surveillance cost estimate of $1 999 029 per year for CA-125 tests alone and $16 194 647 per year with CT scans added.
Conclusions and relevance: CA-125 tests and CT scans are still routinely used for surveillance testing in patients with ovarian cancer, although their benefit has not been proven and their use may have significant implications for patients' quality of life as well as costs.
Conflict of interest statement
The following authors have confirmed that there are no potential conflicts of interest or disclosures to report pertaining to this submission: Katharine M. Esselen, Angel Cronin, Kristen Bixel, David E. Cohn, Michaela Cristea, Jennifer J. Griggs, Charles F. Levenback, Gina Mantia-Smaldone, Ursula A. Matulonis, Joyce C. Niland, Charlotte Sun, and Alexi A. Wright.
Figures


References
-
- SGO - Routine imaging for cancer surveillance. Choosing Wisely. [February 8, 2016]; http://www.choosingwisely.org/clinician-lists/society-gynecologic-oncolo...
-
- Rustin GJ, vdB M. A randomized trial in ovarian cancer (OC) of early treatment of relapse based on CA125 level alone versus delayed treatment based on conventional clinical indicators (MRC OV05/EORTC 55955 trials) J Clin Oncol. 2009;27(18s) abstr 1.
-
- Rustin GJ, van der Burg ME, Griffin CL, et al. Early versus delayed treatment of relapsed ovarian cancer (MRC OV05/EORTC 55955): a randomised trial. Lancet. 2010 Oct 2;376(9747):1155–1163. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

