Electrochemotherapy in the Treatment of Bone Metastases: A Phase II Trial
- PMID: 27443372
- PMCID: PMC5104781
- DOI: 10.1007/s00268-016-3627-6
Electrochemotherapy in the Treatment of Bone Metastases: A Phase II Trial
Abstract
Introduction: Bone metastatic disease is a major cause of pain and decreased quality of life in patients with cancer. In addition to systemic therapy and pain control with narcotic analgesics, standard local treatments include palliation with radiation therapy and surgery. However, 20-30 % of patients do not respond to conventional treatments, increasing the interest in alternative therapies. We present the results of a new minimally invasive technique in the treatment of bone metastases.
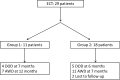
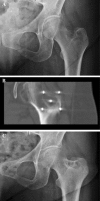
Methods: Twenty-nine patients affected by painful bone metastases were treated with electrochemotherapy (ECT) from July 2009 to July 2011; the mean age was 60 years (range 37-87); 21 patients received a previous ineffective local treatment; the appendicular skeleton was affected in 15 patients while in 14 patients other sites were involved. ECT was performed using the Cliniporator Vitae under fluoroscopy or CT guidance depending on the site of the lesion. Clinical response was assessed using VAS scale and objective tumour response was evaluated according to the MD Anderson criteria for bone metastases.
Results: All patients well tolerated the procedure and no intraoperative or postoperative complications were observed. At a mean follow-up of 7 months, 24 patients were available for evaluation. 84 % of the patients (20 out of 24) referred improvement of pain ≥50 % with reduction of narcotics consumption. Radiographic evaluation after 3 months in 20 evaluable patients, showed "partial response" in 1 patient, "stable disease" in 17 and "progression" in two cases.
Discussion: Results reported in this study demonstrated ECT to be safe and feasible in the treatment of painful bone metastases even when other previous treatments were ineffective. Pain and disease progression control was achieved in the majority of the patients with consequent improvement of quality of life.
Conclusion: ECT should be considered a new feasible tool in the treatment of bone metastases in place or in combination with standard treatments; further developments are required to extend the use of this technique to spine metastases.
Conflict of interest statement
Dr. Bianchi reports grants from IGEA, during the conduction of the study. Dr. Campanacci reports grants from IGEA, during the conduction of the study. Dr. Donati reports grants from IGEA, during the conduction of the study. Dr. Ronchetti is a full-time employee of IGEA.
Figures


References
-
- Manoso MW, Healey JH. cancer. In: DeVita VT, Hellman S, Rosenberg SA, editors. Principles and practice of oncology. 7. Philadelphia: Lippincott Williams and Wilkins; 2005. pp. 2368–2380.
-
- Biermann JS, Holt GE, Lewis VO, Schwartz HS, Yaszemski MJ. Metastatic bone disease: diagnosis, evaluation, and treatment (Review) J Bone Joint Surg Am. 2009;91(6):1518–1530. - PubMed
-
- Testori A, Tosti G, Martinoli C, Spadola G, Cataldo F, Verrecchia F, Baldini F, Mosconi M, Soteldo J, Tedeschi I, Passoni C, Pari C, Di Pietro A, Ferrucci PF. Electrochemotherapy for cutaneous and subcutaneous tumour lesions: a novel therapeutic approach (Review) Dermatol Ther. 2010;23(6):651–661. doi: 10.1111/j.1529-8019.2010.01370.x. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

