Microoxic Niches within the Thylakoid Stroma of Air-Grown Chlamydomonas reinhardtii Protect [FeFe]-Hydrogenase and Support Hydrogen Production under Fully Aerobic Environment
- PMID: 27443604
- PMCID: PMC5074601
- DOI: 10.1104/pp.16.01063
Microoxic Niches within the Thylakoid Stroma of Air-Grown Chlamydomonas reinhardtii Protect [FeFe]-Hydrogenase and Support Hydrogen Production under Fully Aerobic Environment
Abstract
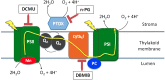
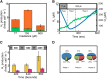
Photosynthetic hydrogen production in the microalga Chlamydomonas reinhardtii is catalyzed by two [FeFe]-hydrogenase isoforms, HydA1 and HydA2, both irreversibly inactivated upon a few seconds exposure to atmospheric oxygen. Until recently, it was thought that hydrogenase is not active in air-grown microalgal cells. In contrast, we show that the entire pool of cellular [FeFe]-hydrogenase remains active in air-grown cells due to efficient scavenging of oxygen. Using membrane inlet mass spectrometry, (18)O2 isotope, and various inhibitors, we were able to dissect the various oxygen uptake mechanisms. We found that both chlororespiration, catalyzed by plastid terminal oxidase, and Mehler reactions, catalyzed by photosystem I and Flavodiiron proteins, significantly contribute to oxygen uptake rate. This rate is considerably enhanced with increasing light, thus forming local anaerobic niches at the proximity of the stromal face of the thylakoid membrane. Furthermore, we found that in transition to high light, the hydrogen production rate is significantly enhanced for a short duration (100 s), thus indicating that [FeFe]-hydrogenase functions as an immediate sink for surplus electrons in aerobic as well as in anaerobic environments. In summary, we show that an anaerobic locality in the chloroplast preserves [FeFe]-hydrogenase activity and supports continuous hydrogen production in air-grown microalgal cells.
© 2016 American Society of Plant Biologists. All rights reserved.
Figures

References
-
- Adamska-Venkatesh A, Krawietz D, Siebel J, Weber K, Happe T, Reijerse E, Lubitz W (2014) New redox states observed in [FeFe] hydrogenases reveal redox coupling within the H-cluster. J Am Chem Soc 136: 11339–11346 - PubMed
-
- Chader S, Hacene H, Agathos SN (2009) Study of hydrogen production by three strains of Chlorella isolated from the soil in the Algerian Sahara. Int J Hydrogen Energy 34: 4941–4946
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

