Tissue-specific gene targeting using CRISPR/Cas9
- PMID: 27443926
- PMCID: PMC5518777
- DOI: 10.1016/bs.mcb.2016.03.004
Tissue-specific gene targeting using CRISPR/Cas9
Abstract
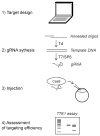
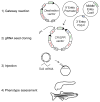
The zebrafish has been a powerful model in forward genetic screens to identify genes essential for organogenesis and embryonic development. Conversely, using reverse genetics to investigate specific gene function requires phenotypic analysis of complete gene inactivation. Despite the availability and efficacy of morpholinos, the lack of tractable and efficient knockout technologies has impeded reverse genetic studies in the zebrafish, particularly in adult animals. The recent development of genome-editing technologies such as CRISPR/Cas9 greatly widened the scope of loss-of-function studies in the zebrafish, allowing for the rapid phenotypic assessment of gene silencing in embryos, the generation of knockout lines, and large-scale reverse genetic screens. Tissue-specific gene inactivation would be ideal for these studies given the caveats of whole-embryo gene silencing, yet spatial control of gene targeting remains a challenge. In this chapter, we focus on tissue-specific gene inactivation using the CRISPR/Cas9 technology. We first explain the rationale for this technique, including some of its potential applications to tackle important biological issues and the inability of current technologies to address these issues. We then present a method to target genes in a tissue-specific manner in the zebrafish. Finally, we discuss technical difficulties and limitations of this method as well as possible future developments.
Keywords: CRISPR/Cas9 technology; Gene knockout; Morpholino; Tissue specificity; gRNAs.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures
References
-
- Barrangou R, Fremaux C, Deveau H, Richards M, Boyaval P, Moineau S, … Horvath P. CRISPR provides acquired resistance against viruses in prokaryotes. Science. 2007;315(5819):1709–1712. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

