Pancreatic cancer biology and genetics from an evolutionary perspective
- PMID: 27444064
- PMCID: PMC5739515
- DOI: 10.1038/nrc.2016.66
Pancreatic cancer biology and genetics from an evolutionary perspective
Abstract
Cancer is an evolutionary disease, containing the hallmarks of an asexually reproducing unicellular organism subject to evolutionary paradigms. Pancreatic ductal adenocarcinoma (hereafter referred to as pancreatic cancer) is a particularly robust example of this phenomenon. Genomic features indicate that pancreatic cancer cells are selected for fitness advantages when encountering the geographic and resource-depleted constraints of the microenvironment. Phenotypic adaptations to these pressures help disseminated cells to survive in secondary sites, a major clinical problem for patients with this disease. In this Review we gather the wide-ranging aspects of pancreatic cancer research into a single concept rooted in Darwinian evolution, with the goal of identifying novel insights and opportunities for study.
Figures


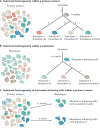

References
-
- Bradford Torrey E. The Writings of Henry David Thoreau. Riverside Press; 1906.
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. - PubMed
-
- Winter JM, et al. Survival after resection of pancreatic adenocarcinoma: results from a single institution over three decades. Ann. Surg. Oncol. 2012;19:169–175. - PubMed
-
- Castellanos E, Berlin J, Cardin DB. Current treatment options for pancreatic carcinoma. Curr. Oncol. Rep. 2011;13:195–205. - PubMed
-
- Yachida S, Iacobuzio-Donahue CA. The pathology and genetics of metastatic pancreatic cancer. Arch. Pathol. Lab. Med. 2009;133:413–422. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

