The clinical relevance of omega-3 fatty acids in the management of hypertriglyceridemia
- PMID: 27444154
- PMCID: PMC4957330
- DOI: 10.1186/s12944-016-0286-4
The clinical relevance of omega-3 fatty acids in the management of hypertriglyceridemia
Abstract
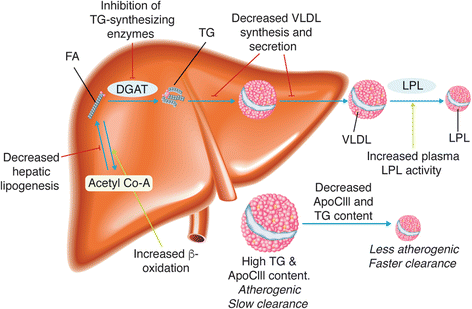
Hypertriglyceridemia (triglycerides > 150 mg/dL) affects ~25 % of the United States (US) population and is associated with increased cardiovascular risk. Severe hypertriglyceridemia (≥ 500 mg/dL) is also a risk factor for pancreatitis. Three omega-3 fatty acid (OM3FA) prescription formulations are approved in the US for the treatment of adults with severe hypertriglyceridemia: (1) OM3FA ethyl esters (OM3EE), a mixture of OM3FA ethyl esters, primarily eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) (Lovaza®, Omtryg™, and generics); (2) icosapent ethyl (IPE), EPA ethyl esters (Vascepa®); and (3) omega-3 carboxylic acids (OM3CA), a mixture of OM3FAs in free fatty acid form, primarily EPA, DHA, and docosapentaenoic acid (Epanova®). At approved doses, all formulations substantially reduce triglyceride and very-low-density lipoprotein levels. DHA-containing formulations may also increase low-density lipoprotein cholesterol. However, this is not accompanied by increased non-high-density lipoprotein cholesterol, which is thought to provide a better indication of cardiovascular risk in this patient population. Proposed mechanisms of action of OM3FAs include inhibition of diacylglycerol acyltransferase, increased plasma lipoprotein lipase activity, decreased hepatic lipogenesis, and increased hepatic β-oxidation. OM3CA bioavailability (area under the plasma concentration-time curve from zero to the last measurable concentration) is up to 4-fold greater than that of OM3FA ethyl esters, and unlike ethyl esters, the absorption of OM3CA is not dependent on pancreatic lipase hydrolysis. All three formulations are well tolerated (the most common adverse events are gastrointestinal) and demonstrate a lack of drug-drug interactions with other lipid-lowering drugs, such as statins and fibrates. OM3FAs appear to be an effective treatment option for patients with severe hypertriglyceridemia.
Keywords: Docosahexaenoic acid; Docosapentaenoic acid; Eicosapentaenoic acid; Hypertriglyceridemia; Omega-3 fatty acids.
Figures
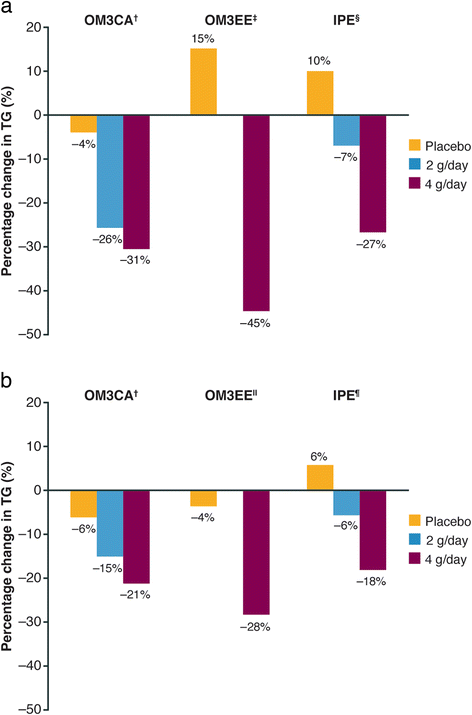
References
-
- Carroll M, Kit B, Lacher D. Trends in elevated triglyceride in adults: United States, 2001–2012. NCHS Data Brief. 2015;198:1–8. - PubMed
-
- Jacobson TA, Ito MK, Maki KC, Orringer CE, Bays HE, Jones PH, McKenney JM, Grundy SM, Gill EA, Wild RA, et al. National Lipid Association recommendations for patient-centered management of dyslipidemia: part 1 - executive summary. J Clin Lipidol. 2014;8:473–488. doi: 10.1016/j.jacl.2014.07.007. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

